结构不同的锰氧化物去除邻苯二酚和对苯二酚效率中的结构-反应关系。
IF 8.1
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
邻苯二酚和对苯二酚是自然环境中广泛存在的羟苯异构体,会对环境造成毒害。锰(Mn)氧化物可通过吸附和氧化降解过程有效去除这些羟苯化合物。在本研究中,我们研究了邻苯二酚和对苯二酚在锰氧化物表面吸附和氧化过程中的结构-反应关系。具体研究了两种广泛存在的氧化锰,包括水合氧化锰(HMO)和隐锰,它们分别由层结构和隧道结构组成。研究了氧化锰结构和环境 pH 条件通过吸附和氧化降解对这些羟苯化合物去除效率的影响。与 HMO 相比,比表面积更大的隐色美兰具有更高的吸附和氧化能力。儿茶酚和对苯二酚的络合机制因其结构引起的反应性差异而不同。与对苯二酚相比,儿茶酚从锰氧化物中还原和溶解的锰更多,同时儿茶酚-C 的 C 损失更高,这表明儿茶酚的反应活性更高。与邻苯二酚和对苯二酚反应后,Mn-氧化物的结构发生了变化:Mn(IV)还原,矿物中相应形成了 Mn(III)和 Mn(II),悬浮液中释放出游离的 Mn2+ 离子。这些见解有助于我们更好地理解和预测羟苯化合物在富含锰氧化物的土壤和废水处理系统中的命运,这些土壤和废水处理系统通过除锰产生锰氧化物,并产生相关的环境毒性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
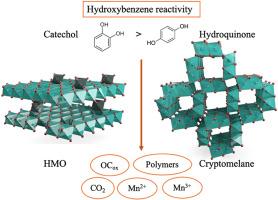
Structure–reactivity relationships in the removal efficiency of catechol and hydroquinone by structurally diverse Mn-oxides
Catechol and hydroquinone are widely present hydroxybenzene isomers in the natural environment that induce environmental toxicities. These hydroxybenzene compounds can be effectively removed by manganese (Mn)-oxides via sorption and oxidative degradation processes. In the present study, we investigated the structure–reactivity relationships in the sorption and oxidation of catechol and hydroquinone on Mn-oxide surfaces. Two widely present Mn-oxides, including hydrous Mn oxide (HMO) and cryptomelane, comprised of layer and tunnel structures, respectively, are specifically studied. Effects of Mn-oxide structures and environmental pH conditions on the removal efficiency of these hydroxybenzene compounds, via sorption and oxidative degradation, are investigated. Cryptomelane, which has a higher specific surface area than HMO, possesses a higher sorption and oxidation capacity. The complexation mechanisms of catechol and hydroquinone vary due to their structure-induced difference in reactivity. Catechol reduced and dissolved more Mn from Mn-oxides than hydroquinone, accompanied by a higher C loss of catechol-C, suggesting a higher reactivity of catechol. Structural changes occurred in the Mn-oxides resulting from reaction with catechol and hydroquinone: reduction of Mn(IV), corresponding formation of Mn(III) and Mn(II) in the mineral, and free Mn2+ ions released into the suspension. These insights could help us better understand and predict the fate of hydroxybenzene compounds in Mn-oxide-rich soils and wastewater treatment systems that generate Mn-oxides via Mn removal and the associated environmental toxicity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemosphere
环境科学-环境科学
CiteScore
15.80
自引率
8.00%
发文量
4975
审稿时长
3.4 months
期刊介绍:
Chemosphere, being an international multidisciplinary journal, is dedicated to publishing original communications and review articles on chemicals in the environment. The scope covers a wide range of topics, including the identification, quantification, behavior, fate, toxicology, treatment, and remediation of chemicals in the bio-, hydro-, litho-, and atmosphere, ensuring the broad dissemination of research in this field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


