镁功能化颗粒煤矸石的砷封存作用:镁的影响和对砷吸附机制的认识。
IF 8.1
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
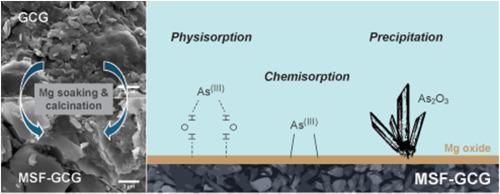
利用天然废料去除溶液中的无机污染物为促进资源循环利用和可持续发展提供了一种新方法。这项研究通过基于镁浸泡和煅烧的表面改性,提高了原煤煤矸石对亚砷酸(As[III])的适度去除能力。批量实验表明,改性后 As(III) 的去除率从 39.8% 提高到 89.9%,与初始 pH 值无关。根据 Langmuir 模型估算,改性煤矸石的最大吸附容量为 0.979 mg/g。理化分析证实,改性增加了煤矸石的表面积、孔体积和尺寸。此外,扫描电子显微镜以及随后的 TEM 和 SAED 分析确定了改性煤矸石上的针状三氧化二砷 (As2O3),从而提高了 As(III) 的去除率。吸附动力学的变化暗示了沉淀的产生,这可能是由于 As(III)吸附到 Mg(OH)2 上形成的 AsO3 聚合物链,而 MgO 在水溶液条件下水合生成了 AsO3 聚合物链。我们的研究结果表明,煤矸石在开发可持续的废物回收方法方面具有潜在的应用价值。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Arsenic sequestration by granular coal gangue functionalized with magnesium: Effects of magnesium and insight of arsenic sorption mechanisms
Leveraging natural waste materials for inorganic contaminant removal in solution offers a novel approach to boost resource recycling and foster sustainable development by enhancing waste use. This research advanced the modest arsenite (As[III]) removal capacity of raw coal gangue through a magnesium-soaking and calcination-based surface modification. Batch experiments showed As(III) removal efficiency was improved from 39.8% to 89.9% after modification, independent of initial pH levels. The Langmuir model estimated the maximum sorption capacity of 0.979 mg/g for the modified coal gangue. Physicochemical analyses confirmed that the modification increased the surface area, pore volume and size of the coal gangue. Furthermore, SEM, and subsequent TEM and SAED analyses identified acicular arsenic trioxide (As2O3) on the modified gangue, enhancing As(III) removal. Variations in sorption kinetics hinted at precipitation, likely due to AsO3 polymer chains formed by As(III)'s sorption onto Mg(OH)2, created from MgO hydration in aqueous conditions. Our findings show that coal gangue has potential applications in the development of sustainable methods for waste recycling.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemosphere
环境科学-环境科学
CiteScore
15.80
自引率
8.00%
发文量
4975
审稿时长
3.4 months
期刊介绍:
Chemosphere, being an international multidisciplinary journal, is dedicated to publishing original communications and review articles on chemicals in the environment. The scope covers a wide range of topics, including the identification, quantification, behavior, fate, toxicology, treatment, and remediation of chemicals in the bio-, hydro-, litho-, and atmosphere, ensuring the broad dissemination of research in this field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


