利用混合球体和微流控平台模拟肿瘤与免疫的相互作用,研究黑色素瘤中肿瘤相关巨噬细胞的极化。
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
肿瘤相关巨噬细胞(TAMs)作为肿瘤微环境(TME)的关键组成部分,在环境线索的作用下表现出表型可塑性,从而极化为促炎性 M1 表型或免疫抑制性 M2 表型。虽然 TAM 因其在癌细胞的发生、发展、转移和免疫调节中的重要作用而被广泛研究,但人们对癌细胞的转移潜能如何影响 TAM 在 TME 内的极化的了解还很有限。在这里,我们利用由小鼠黑色素瘤细胞和巨噬细胞组成的三维杂交系统开发了一个微型化的TME模型,旨在研究具有各种转移潜能的癌细胞和巨噬细胞在TME内的相互作用。该模型中球形细胞体积的增大与癌细胞存活率的降低有关。对巨噬细胞表面标志物表达和细胞因子分泌的研究表明,球形体大小和癌细胞转移潜能会影响不同TME的发展。此外,该研究还采用了配备捕获系统和混合球体的高通量微流控平台来模拟复杂TMEs的肿瘤免疫系统,并与传统的三维培养模型进行比较分析。这项研究通过模拟肿瘤免疫系统,深入了解了不同异质性黑色素瘤中的 TAM 极化情况,可用于免疫肿瘤学研究、药物筛选和个性化治疗。意义说明:本研究开发了一种三维混合球形系统,旨在模拟肿瘤与免疫的相互作用,详细分析黑色素瘤细胞的转移潜能如何影响肿瘤相关巨噬细胞(TAM)的极化。通过利用微流体平台,我们能够在连续流条件下复制和研究肿瘤微环境(TME)中复杂的肿瘤免疫系统。我们的模型在高通量药物筛选和个性化医疗应用方面具有巨大潜力,为推进癌症研究和治疗策略提供了一种多功能工具。本文章由计算机程序翻译,如有差异,请以英文原文为准。
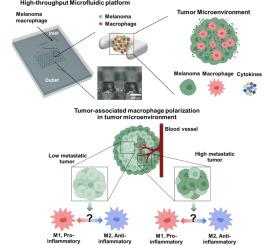
Modeling tumor-immune interactions using hybrid spheroids and microfluidic platforms for studying tumor-associated macrophage polarization in melanoma
Tumor-associated macrophages (TAMs), as key components of tumor microenvironment (TME), exhibit phenotypic plasticity in response to environmental cues, causing polarization into either pro-inflammatory M1 phenotypes or immunosuppressive M2 phenotypes. Although TAM has been widely studied for its crucial involvement in the initiation, progression, metastasis, and immune regulation of cancer cells, there have been limited attempts to understand how the metastatic potentials of cancer cells influence TAM polarization within TME. Here, we developed a miniaturized TME model using a 3D hybrid system composed of murine melanoma cells and macrophages, aiming to investigate interactions between cancer cells exhibiting various metastatic potentials and macrophages within TME. The increase in spheroid size within this model was associated with a reduction in cancer cell viability. Examining macrophage surface marker expression and cytokine secretion indicated the development of diverse TMEs influenced by both spheroid size and the metastatic potential of cancer cells. Furthermore, a high-throughput microfluidic platform equipped with trapping systems and hybrid spheroids was employed to simulate the tumor-immune system of complex TMEs and for comparative analysis with traditional 3D culture models. This study provides insight into TAM polarization in melanoma with different heterogeneities by modeling cancer-immune systems, which can be potentially employed for immune-oncology research, drug screening, and personalized therapy.
Statement of significance
This study presents the development of a 3D hybrid spheroid system designed to model tumor-immune interactions, providing a detailed analysis of how melanoma cell metastatic potential influences tumor-associated macrophage (TAM) polarization. By utilizing a microfluidic platform, we are able to replicate and investigate the complex tumor-immune system of the tumor microenvironments (TMEs) under continuous flow conditions. Our model holds significant potential for high-throughput drug screening and personalized medicine applications, offering a versatile tool for advancing cancer research and treatment strategies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


