杜松水提取物对大鼠急性和亚急性毒性的毒理学评估。
IF 2.6
4区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
杜松(Juniperus oxycedrus L.)在治疗消化系统异常的草药疗法中有着丰富的历史背景。然而,尚未对其潜在的毒性作用进行过全面评估。本次调查旨在评估土荆皮水提取物(AEJO)的急性和亚急性毒性。AEJO 采用摩洛哥传统方法,通过煎煮植物的芳香部分制备而成。分别在小鼠和大鼠身上进行了急性和亚急性毒性试验。急性毒性试验表明,即使在 5000 毫克/千克的高剂量下,提取物也不会产生毒性。在亚急性研究中,没有观察到毒性或死亡迹象,所有大鼠的进食量和饮水量也没有明显偏差。不过,1000 毫克/千克和 2000 毫克/千克剂量下的动物体重出现了显著下降。服用 AEJO 会减少血小板数量,升高丙氨酸氨基转移酶和碱性磷酸酶水平,降低白蛋白水平。组织学检查显示,尽管肌酐升高,但肾实质正常。它还显示了双核和肝细胞空泡化。结果表明,AEJO 的耐受性很强,但反复使用会影响肝细胞和肾脏。因此,在将这种植物用于临床研究之前,需要进行更多的分析,如亚慢性、慢性和神经毒性研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
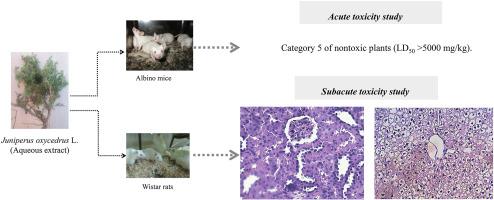
Toxicological assessment of the aqueous extract of Juniperus oxycedrus L. on acute and subacute toxicities in rats
Juniperus oxycedrus L. (J. oxycedrus) has a rich historical background in herbal remedies to treating digestive system abnormalities. However, no comprehensive evaluation of its potential toxic effects has been conducted. The current investigation aimed to evaluate the acute and subacute toxicity of an aqueous extract of J. oxycedrus (AEJO). AEJO was prepared by the conventional Moroccan methods by decoction the arial part of the plant. The acute and subacute toxicity tests were conducted in mice and rats, respectively. Acute toxicity tests showed that the extract was not toxic even at high doses of 5000 mg/kg. In the subacute study, no detectable indications of toxicity or mortality were observed and there were no notable deviations in food intake or water consumption among all rats. However, changes in body weight of animals treated with 1000 and 2000 mg/kg underwent a significant decrease. AEJO administration decreased platelet number, elevated levels of alanine aminotransferase and alkaline phosphatase, and reduced albumin levels. Histological examination revealed normal renal parenchyma despite increased creatinine. It also showed binucleation, and hepatocyte vacuolation. The results indicate that AEJO has considerable tolerance for consumption, but repeated use can affect hepatocytes and kidneys. Therefore, additional analyses, such as subchronic, chronic, and neurotoxic studies, are required before using this plant in clinical research.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Toxicon
医学-毒理学
CiteScore
4.80
自引率
10.70%
发文量
358
审稿时长
68 days
期刊介绍:
Toxicon has an open access mirror Toxicon: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review. An introductory offer Toxicon: X - full waiver of the Open Access fee.
Toxicon''s "aims and scope" are to publish:
-articles containing the results of original research on problems related to toxins derived from animals, plants and microorganisms
-papers on novel findings related to the chemical, pharmacological, toxicological, and immunological properties of natural toxins
-molecular biological studies of toxins and other genes from poisonous and venomous organisms that advance understanding of the role or function of toxins
-clinical observations on poisoning and envenoming where a new therapeutic principle has been proposed or a decidedly superior clinical result has been obtained.
-material on the use of toxins as tools in studying biological processes and material on subjects related to venom and antivenom problems.
-articles on the translational application of toxins, for example as drugs and insecticides
-epidemiological studies on envenoming or poisoning, so long as they highlight a previously unrecognised medical problem or provide insight into the prevention or medical treatment of envenoming or poisoning. Retrospective surveys of hospital records, especially those lacking species identification, will not be considered for publication. Properly designed prospective community-based surveys are strongly encouraged.
-articles describing well-known activities of venoms, such as antibacterial, anticancer, and analgesic activities of arachnid venoms, without any attempt to define the mechanism of action or purify the active component, will not be considered for publication in Toxicon.
-review articles on problems related to toxinology.
To encourage the exchange of ideas, sections of the journal may be devoted to Short Communications, Letters to the Editor and activities of the affiliated societies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


