通窍活血汤通过cAMP/PKA/CREB途径促进血管性痴呆大鼠突触重塑
IF 6.7
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
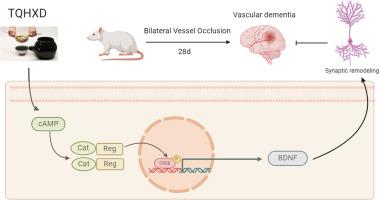
背景介绍通窍活血汤(TQHXD)是一种广泛用于治疗血管性痴呆(VD)的传统中药配方。虽然该方具有良好的临床疗效,但其治疗血管性痴呆的具体分子机制仍不清楚:本研究旨在阐明 TQHXD 的神经保护机制,为 VD 的临床治疗提供科学依据:方法:采用超高效液相色谱法(UPLC)和气相色谱法(GC)对TQHXD的化学成分进行定性分析。网络药理学预测了 TQHXD 对 VD 的潜在保护机制。通过双侧血管闭塞(2-VO)建立了大鼠 VD 模型,并使用氧-葡萄糖剥夺/再灌注(OGD/R)模型诱导海马神经元细胞损伤。体内实验评估了大鼠脑血流量、学习和记忆能力、海马神经元形态、树突长度、树突棘密度和突触数量的变化。我们检测了海马区突触重塑相关蛋白和通路蛋白的表达。体外试验评估了细胞活力、凋亡、活性氧(ROS)水平以及突触重塑相关蛋白和信号通路的表达:结果:在TQHXD中发现了多种活性成分。KEGG富集分析表明,TQHXD对VD的治疗作用可能与cAMP信号通路有关。用 TQHXD 治疗可明显改善 VD 大鼠的学习和记忆能力,改善海马形态,增加树突长度、树突棘密度和突触数量。此外,TQHXD 还能在体外提高细胞活力、减少细胞凋亡和降低细胞内 ROS 水平。Western印迹、免疫荧光和酶联免疫吸附试验结果共同表明,TQHXD能在体内和体外上调突触重塑相关蛋白和通路相关蛋白的表达:结论:TQHXD可能通过激活cAMP/PKA/CREB通路,促进海马神经元的突触重塑,从而治疗VD。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Tong-Qiao-Huo-Xue Decoction promotes synaptic remodeling via cAMP/PKA/CREB pathway in vascular dementia rats
Background
Tong-Qiao-Huo-Xue Decoction (TQHXD) is a traditional Chinese medicinal formula widely used in the treatment of vascular dementia (VD). Although it has demonstrated good clinical efficacy, the specific molecular mechanisms underlying its therapeutic effects on VD remain unclear.
Objective
This study aimed to elucidate the neuroprotective mechanisms of TQHXD to provide a scientific basis for the clinical treatment of VD.
Methods
The chemical components of TQHXD were qualitatively analyzed using ultra-performance liquid chromatography (UPLC) and gas chromatography (GC). Network pharmacology predicted the potential protective mechanisms of TQHXD against VD. A rat model of VD was established through bilateral vessel occlusion (2-VO), and an oxygen-glucose deprivation/reperfusion (OGD/R) model was used to induce damage to neuronal cells of the hippocampus. In vivo experiments assessed changes in cerebral blood flow, learning and memory capabilities, hippocampal neuronal morphology, dendritic length, dendritic spine density, and synapse number in rats. We examined the expression of synaptic remodeling-related proteins and pathway proteins in the hippocampal region. In vitro assays evaluated cell viability, apoptosis, reactive oxygen species (ROS) levels, and expression of synaptic remodeling-related proteins and signaling pathway.
Results
Multiple active components were identified in TQHXD. KEGG enrichment analysis suggested that the therapeutic effects of TQHXD on VD may be related to the cAMP signaling pathway. Treatment with TQHXD significantly improved learning and memory performance in VD rats, improved hippocampus morphology, and increased dendritic length, dendritic spine density, and number of synapses. Furthermore, TQHXD improved cell viability, reduced apoptosis, and decreased intracellular ROS levels in vitro. Western blotting, immunofluorescence, and enzyme-linked immunosorbent assay results collectively demonstrated that TQHXD upregulated the expression of synaptic remodeling-related proteins and pathway-related proteins both in vivo and in vitro.
Conclusions
TQHXD treated VD by promoting synaptic remodeling in hippocampal neurons, likely through activation of the cAMP/PKA/CREB pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


