青蒿素通过靶向 FDFT1 诱导乳腺癌细胞凋亡,并抑制乳腺癌患者衍生器官组织的生长。
IF 6.7
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
青蒿素(ATT)是一种具有抗乳腺癌活性的天然生物活性化合物。然而,青蒿素对乳腺癌的直接靶点和临床疗效仍不清楚。本研究旨在确定青蒿素抗乳腺癌的靶蛋白及其作用机制。此外,本研究还采用患者衍生的器官组织(PDOs)来评估ATT对乳腺癌的临床疗效。本文应用分子对接、分子动力学模拟、细胞热转移试验(CETSA)结合Western印迹、表面等离子体共振(SPR)等方法证实了ATT在乳腺癌细胞中的相互作用靶点。通过生物信息学分析、Western 印迹、流式细胞术、质粒构建和慢病毒感染、染色质免疫沉淀(ChIP)检测和实时定量 PCR(RT-qPCR),揭示了 ATT 治疗乳腺癌的潜在机制。我们建立了PDOs来评估ATT对乳腺癌的临床治疗效果。我们发现 ATT 与乳腺癌细胞中的二磷酸法尼基转移酶 1(FDFT1)相互作用。敲除 FDFT1 可诱导乳腺癌细胞中 NEDD4 的表达和凋亡。过表达FDFT1可挽救ATT诱导的细胞凋亡,而干扰FDFT1的表达可降低乳腺癌细胞核中RelA(NF-κB p65亚基)的水平。敲除 FDFT1 可通过调节 TNFR1/NF-κB 通路诱导 NEDD4 的表达。过表达 FDFT1 可逆转 ATT 诱导的 TNFR1/NF-κB/NEDD4 通路在乳腺癌细胞中的激活。有趣的是,ChIP 检测和 RT-qPCR 发现 p65 可以调控 NEDD4 的转录。此外,ATT 对不同病理亚型的乳腺癌 PDO 的生长具有广谱的抑制作用,与传统化疗药物相比,具有良好的安全性。综上所述,该研究表明 ATT 可通过调节 TNFR1/NF-κB/NEDD4 通路靶向 FDFT1 诱导乳腺癌细胞凋亡,并抑制乳腺癌 PDO 的生长,从而支持 ATT 成为治疗乳腺癌的有效和潜在候选药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
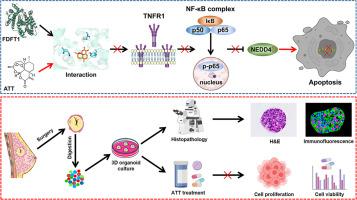
Artemisitene induces apoptosis of breast cancer cells by targeting FDFT1 and inhibits the growth of breast cancer patient-derived organoids
Artemisitene (ATT) is a natural bioactive compound with anti-breast cancer activity. However, the direct target and clinical efficacy of ATT on breast cancer are still unclear. The current study aimed to identify the target protein and underlying mechanism of ATT in anti-breast cancer. Moreover, patient-derived organoids (PDOs) were employed to assess the clinical efficacy of ATT on breast cancer. Herein, molecular docking, molecular dynamics simulation, cellular thermal shift assay (CETSA) combined with Western blot, surface plasmon resonance (SPR) were applied to confirm the interactional target of ATT in breast cancer cells. Bioinformatics analysis, Western blot, flow cytometry, plasmid construction and lentivirus infection, chromatin immunoprecipitation (ChIP) assay, and quantitative real-time PCR (RT-qPCR) were performed to reveal the potential mechanism of ATT in treating breast cancer. PDOs were established to evaluate the clinical therapeutic efficiency of ATT on breast cancer. We found that ATT interacted with Farnesyl-diphosphate farnesyltransferase 1 (FDFT1) in breast cancer cells. Knockdown of FDFT1 induced NEDD4 expression and apoptosis in breast cancer cells. Overexpression of FDFT1 could rescue ATT-induced apoptosis, while interfering with FDFT1 expression decreased the level of RelA (NF-κB p65 subunit) in the nucleus in breast cancer cells. Knockdown of FDFT1 induced NEDD4 expression by regulating TNFR1/NF-κB pathway. Overexpression of FDFT1 could reverse the activation of ATT-induced TNFR1/NF-κB/NEDD4 pathway in breast cancer cells. Interestingly, the ChIP assay and RT-qPCR revealed that p65 could regulate NEDD4 transcription. Furthermore, ATT exhibited a broad-spectrum inhibitory effect on the growth of breast cancer PDOs with different pathological subtypes, and showed an excellent safety profile in comparison with that of conventional chemotherapy drugs. In summary, this work demonstrated that ATT targets FDFT1 to induce apoptosis of breast cancer cells through regulating TNFR1/NF-κB/NEDD4 pathway and suppresses breast cancer PDOs growth, which supported ATT as an effective and potential drug candidate for breast cancer treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


