从大戟科植物 helioscopia 中提取出具有抗炎活性的ent-Abietane 型内酯。
IF 3.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
通过生物活性指导分离法,从E. helioscopia提取的抗炎馏分中分离出了Euphohelinodes D-I(1-6)、六种以前未报道过的ent-abietane内酯以及两种已知的类似物(7和8)。通过光谱数据解读、单晶 X 射线衍射和 ECD 分析,对它们的结构进行了表征。通过测量这些化合物对 LPS 刺激的 RAW264.7 巨噬细胞产生的 NO 的抑制作用,评估了它们的抗炎活性。活性最高的候选化合物 euphohelinode H(5)对 NO 生成具有较好的抑制活性,其 IC50 值为 30.23 ± 2.33 μM。进一步研究发现,5 通过 NF-κB 信号通路明显抑制了 iNOS 和 COX-2 的表达。本文章由计算机程序翻译,如有差异,请以英文原文为准。
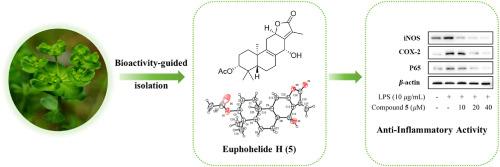
ent-Abietane-type lactones with anti-inflammatory activity from Euphorbia helioscopia
Euphohelinodes D–I (1–6), six previously unreported ent-abietane lactones, along with two known analogues (7 and 8), were isolated from the anti-inflammatory fraction extracted from E. helioscopia by a bioactivity-guided isolation. Their structures were characterized using a combination of spectroscopic data interpretation, single-crystal X-ray diffraction and ECD analysis. The anti-inflammatory activity of these compounds was evaluated by measuring their inhibitory effects on NO production in LPS-stimulated RAW264.7 macrophages. The most active candidate, euphohelinode H (5), had better inhibitory activity against NO production with an IC50 value of 30.23 ± 2.33 μM. Further study revealed that 5 significantly suppressed the expressions of iNOS and COX-2 through the NF-κB signaling pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


