神经肽 Y Y2 受体在急性和慢性疼痛和瘙痒中的作用。
IF 2.7
3区 医学
Q3 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
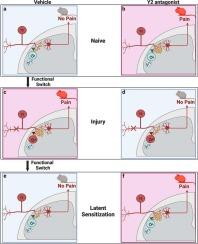
疼痛和瘙痒是由脊髓背角(DH)浅层的多种神经肽及其受体调节的。神经肽 Y(NPY)通常在 DH 神经元上表达,但不在感觉神经元上表达。相比之下,Npy2r 受体(Y2)表达于感觉神经元的中枢和外周末梢,但不表达于 DH 神经元。神经电生理切片记录表明,Y2 选择性激动剂会抑制感觉神经元释放脊髓神经递质。然而,行为药理学研究表明,Y2 激动剂对痛觉的改变微乎其微,即使在损伤后也是如此。Y2-拮抗剂 BIIE0246 在行为学作用上的其他差异--关于代痛觉或抗痛觉的报告--现已得到解决。在正常状态下,脊髓定向(鞘内)给药 BIIE0246 会引起持续的痛觉、感觉刺激过敏和厌恶。相反,在神经损伤和炎症的情况下,鞘内注射 BIIE024 不仅能降低机械和热超敏反应,还能降低疼痛的情感维度(条件性位置偏好)。在慢性疼痛潜敏化模型中给药时,BIIE0246能显著恢复类似疼痛的行为。我们认为,组织或神经损伤会诱导 G 蛋白切换 NPY-Y2 信号的作用,从幼稚状态下的抗痛觉到损伤状态下机械和热超敏痛觉的抑制,然后再切换回抗痛觉以保持 LS 处于缓解状态。这一模型阐明了Y2研究的药物治疗潜力,并指出可以利用Y2拮抗剂开发一种新的非阿片类药物疗法,用于治疗未发展为LS的慢性疼痛患者。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Neuropeptide Y Y2 receptors in acute and chronic pain and itch
Pain and itch are regulated by a diverse array of neuropeptides and their receptors in superficial laminae of the spinal cord dorsal horn (DH). Neuropeptide Y (NPY) is normally expressed on DH neurons but not sensory neurons. By contrast, the Npy2r receptor (Y2) is expressed on the central and peripheral terminals of sensory neurons but not on DH neurons. Neurophysiological slice recordings indicate that Y2-selective agonists inhibits spinal neurotransmitter release from sensory neurons. However, behavioral pharmacology studies indicate that Y2 agonists exert minimal changes in nociception, even after injury. Additional discrepancies in the behavioral actions of the Y2-antagonist BIIE0246 – reports of either pronociception or antinociception – have now been resolved. In the normal state, spinally-directed (intrathecal) administration of BIIE0246 elicits ongoing nociception, hypersensitivity to sensory stimulation, and aversion. Conversely, in the setting of nerve injury and inflammation, intrathecal BIIE024 reduced not only mechanical and thermal hypersensitivity, but also a measure of the affective dimension of pain (conditioned place preference). When administered in chronic pain models of latent sensitization, BIIE0246 produced a profound reinstatement of pain-like behaviors. We propose that tissue or nerve injury induces a G protein switch in the action of NPY-Y2 signaling from antinociception in the naïve state to the inhibition of mechanical and heat hyperalgesia in the injured state, and then a switch back to antinociception to keep LS in a state of remission. This model clarifies the pharmacotherapeutic potential of Y2 research, pointing to the development of a new non-opioid pharmacotherapy for chronic pain using Y2 antagonists in patients who do not develop LS.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Neuropeptides
医学-内分泌学与代谢
CiteScore
5.40
自引率
6.90%
发文量
55
审稿时长
>12 weeks
期刊介绍:
The aim of Neuropeptides is the rapid publication of original research and review articles, dealing with the structure, distribution, actions and functions of peptides in the central and peripheral nervous systems. The explosion of research activity in this field has led to the identification of numerous naturally occurring endogenous peptides which act as neurotransmitters, neuromodulators, or trophic factors, to mediate nervous system functions. Increasing numbers of non-peptide ligands of neuropeptide receptors have been developed, which act as agonists or antagonists in peptidergic systems.
The journal provides a unique opportunity of integrating the many disciplines involved in all neuropeptide research. The journal publishes articles on all aspects of the neuropeptide field, with particular emphasis on gene regulation of peptide expression, peptide receptor subtypes, transgenic and knockout mice with mutations in genes for neuropeptides and peptide receptors, neuroanatomy, physiology, behaviour, neurotrophic factors, preclinical drug evaluation, clinical studies, and clinical trials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


