从诱导多能干细胞分化出内脏感觉神经节器官组织。
IF 36.1
1区 生物学
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
从诱导多能干细胞(iPS)生成内脏感觉神经元(VSN)的能力可能有助于深入了解肠道-神经-大脑轴是如何参与神经系统疾病的。我们建立了一种分化源自人类iPS细胞的内脏感觉神经节器官组织(VSGOs)的方案。VSGOs表现出典型的VSN标记,单细胞RNA测序揭示了VSGOs与原生VSN一致的异源分子特征和发育轨迹。我们在微流控装置上整合了 VSGOs 与人类结肠器官组织,并将这种芯片轴模型应用于阿尔茨海默病。我们的研究结果表明,VSN 可能是一种潜在的介质,能以 APOE4 和 LRP1 依赖性方式将肠道衍生的淀粉样蛋白和 tau 传播到大脑。此外,我们的研究方法还扩展到了源自患者的 iPS 细胞,这些细胞与临床数据有很强的相关性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
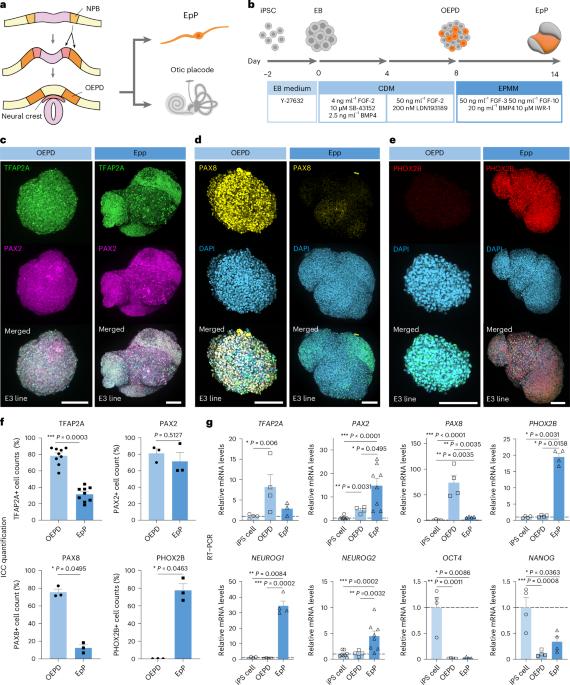
Differentiating visceral sensory ganglion organoids from induced pluripotent stem cells
The ability to generate visceral sensory neurons (VSN) from induced pluripotent stem (iPS) cells may help to gain insights into how the gut–nerve–brain axis is involved in neurological disorders. We established a protocol to differentiate human iPS-cell-derived visceral sensory ganglion organoids (VSGOs). VSGOs exhibit canonical VSN markers, and single-cell RNA sequencing revealed heterogenous molecular signatures and developmental trajectories of VSGOs aligned with native VSN. We integrated VSGOs with human colon organoids on a microfluidic device and applied this axis-on-a-chip model to Alzheimer’s disease. Our results suggest that VSN could be a potential mediator for propagating gut-derived amyloid and tau to the brain in an APOE4- and LRP1-dependent manner. Furthermore, our approach was extended to include patient-derived iPS cells, which demonstrated a strong correlation with clinical data. A protocol for differentiating visceral sensory ganglion organoids from induced pluripotent stem cells allows the establishment of an in vitro model for the gut–visceral nerve–brain axis and study of the propagation of pathogenic proteins involved in Alzheimer’s disease along the vagus nerve.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Methods
生物-生化研究方法
CiteScore
58.70
自引率
1.70%
发文量
326
审稿时长
1 months
期刊介绍:
Nature Methods is a monthly journal that focuses on publishing innovative methods and substantial enhancements to fundamental life sciences research techniques. Geared towards a diverse, interdisciplinary readership of researchers in academia and industry engaged in laboratory work, the journal offers new tools for research and emphasizes the immediate practical significance of the featured work. It publishes primary research papers and reviews recent technical and methodological advancements, with a particular interest in primary methods papers relevant to the biological and biomedical sciences. This includes methods rooted in chemistry with practical applications for studying biological problems.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


