开发负载红霉素的聚乳酸乙二醛(PLGA)纳米颗粒,以提高药物疗效和持续释放能力,防止细菌感染和生物膜形成。
IF 3.3
3区 医学
Q3 IMMUNOLOGY
引用次数: 0
摘要
细菌感染是败血症的常见病因,通常会导致很高的患者死亡率。由于细菌对许多现有药物产生耐药性,治疗这类感染具有挑战性。红霉素(Ery)是一种大环内酯类抗生素,用于治疗细菌感染,但有报道称存在耐药性。最近,合成的聚乳酸共聚乙醇酸(PLGA)聚合物纳米粒子(NPs)显示出了更好的给药特性和生物相容性。本研究采用 O/w 乳化扩散法合成了 PLGA-Ery NPs,粒径为 159 ± 23 nm,封装效率为 71.89%。与纯药物相比,PLGA-Ery NPs 对大肠杆菌、金黄色葡萄球菌和绿脓杆菌的 MIC 和抗菌效力分别提高了 1.5、2.1 和 1.5 倍。扫描电子显微镜显示,PLGA-Ery NPs 会破坏细菌的细胞壁。此外,根据荧光显微镜和 MTT 试验的测定,在玻璃表面涂覆 PLGA-Ery NPs 能有效抑制铜绿假单胞菌形成生物膜(>90%)。这项研究表明,PLGA-Ery NPs 可以提高红霉素的效率,抑制铜绿假单胞菌的生长和生物膜的形成。这种聚合物纳米粒子药物纳米制剂具有抗菌和作为医疗器械表面涂层的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
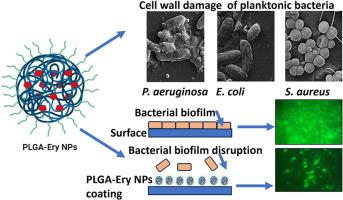
Development of erythromycin loaded PLGA nanoparticles for improved drug efficacy and sustained release against bacterial infections and biofilm formation
Bacterial infections are a common cause of sepsis, often leading to high patient mortality. Such infections are challenging to treat due to bacterial resistance to many existing drugs. Erythromycin (Ery) is a macrolide antibiotic used against bacterial infections with reported resistance. Recently, synthetic poly-lactide co-glycolic acid (PLGA) polymer nanoparticles (NPs) have displayed improved drug delivery characteristics and biocompatibility. In this study, PLGA-Ery NPs were synthesized by the o/w emulsion diffusion method, having a particle size of 159 ± 23 nm and displayed 71.89 % of encapsulation efficiency. The PLGA-Ery NPs showed 1.5, 2.1 and 1.5-fold improved MIC and antibacterial efficacy against E. coli, S. aureus, and P. aeruginosa, respectively than the pure drug. As illustrated by scanning electron microscopy, PLGA-Ery NPs caused damage to the bacterial cell walls. Furthermore, a surface coating with PLGA-Ery NPs on a glass surface showed efficient inhibition (>90 %) of the biofilm formation by P. aeruginosa, as determined by fluorescence microscopy and MTT assay. This study demonstrates that PLGA-Ery NPs can increase the efficiency of erythromycin and can suppress the growth and biofilm formation of P. aeruginosa. Such polymeric nanoparticles drug nanoformulations have potential as an antimicrobial and as a surface coating for medical devices.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Microbial pathogenesis
医学-免疫学
CiteScore
7.40
自引率
2.60%
发文量
472
审稿时长
56 days
期刊介绍:
Microbial Pathogenesis publishes original contributions and reviews about the molecular and cellular mechanisms of infectious diseases. It covers microbiology, host-pathogen interaction and immunology related to infectious agents, including bacteria, fungi, viruses and protozoa. It also accepts papers in the field of clinical microbiology, with the exception of case reports.
Research Areas Include:
-Pathogenesis
-Virulence factors
-Host susceptibility or resistance
-Immune mechanisms
-Identification, cloning and sequencing of relevant genes
-Genetic studies
-Viruses, prokaryotic organisms and protozoa
-Microbiota
-Systems biology related to infectious diseases
-Targets for vaccine design (pre-clinical studies)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


