另一种胰岛素通过整合神经内分泌信号和 SERCA2a 活性来调节心肌细胞的钙处理。
IF 4.9
2区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
摘要
钙(Ca2+)失调是心血管疾病的一个标志性特征。细胞内 Ca2+ 的调节对心脏的正常功能至关重要,它由肌浆/内质网 Ca2+ ATPase(SERCA2a)控制。另一种调节蛋白(ALN)是一种新发现的心肌细胞表达的 SERCA2a 抑制剂,这表明心肌细胞的 Ca2+ 处理比以前认识到的更为复杂。为了研究 ALN 在心肌细胞中的作用,我们产生了 ALN 空断小鼠(基因敲除,KO),发现与野生型(WT)小鼠相比,这些动物的心肌细胞显示出增强的 Ca2+ 循环和收缩力,表明 SERCA2a 活性增强。体外和体内研究表明,ALN通过丝氨酸19(S19)上的磷酸化进行翻译后修饰,表明这有助于其调节SERCA2a的能力。对 ALN-WT、磷酸化缺陷 ALN(S19A)或磷酸拟态 ALN(S19D)的免疫沉淀和 FRET 分析表明,S19 磷酸化改变了 SERCA2a 与 ALN 的相互作用,导致其抑制作用减弱。腺相关病毒介导的 ALN-WT 或磷酸化突变 ALN-S19A/D 在 ALN KO 小鼠中的传递表明,与 ALN-WT 和 ALN-S19D 表达细胞相比,心肌细胞特异性表达磷酸化缺陷 ALN-S19A 会导致 SERCA2a 抑制作用增强,其特征是细胞质 Ca2+ 清除率降低,这进一步证明了这种磷酸化事件在 ALN 控制 SERCA2a 调节中的作用。在 Gαq 激动剂(血管紧张素 II、内皮素-1、苯肾上腺素)作用下,心肌细胞中的 ALN 磷酸化水平显著增加,Gαq 介导的 ALN 磷酸化在 WT 而非 ALN KO 小鼠的心肌细胞中转化为增加的 Ca2+ 循环。总之,这些结果表明,ALN 通过整合神经内分泌信号和 SERCA2a 活性,对心肌细胞中的 Ca2+ 处理进行独特的调节。本文章由计算机程序翻译,如有差异,请以英文原文为准。
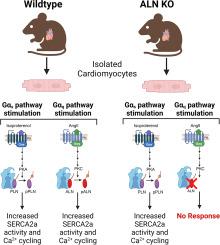
Another-regulin regulates cardiomyocyte calcium handling via integration of neuroendocrine signaling with SERCA2a activity
Calcium (Ca2+) dysregulation is a hallmark feature of cardiovascular disease. Intracellular Ca2+ regulation is essential for proper heart function and is controlled by the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA2a). Another-regulin (ALN) is a newly discovered cardiomyocyte-expressed SERCA2a inhibitor, suggesting cardiomyocyte Ca2+-handling is more complex than previously appreciated. To study the role of ALN in cardiomyocytes, we generated ALN null mice (knockout, KO) and found that cardiomyocytes from these animals displayed enhanced Ca2+ cycling and contractility compared to wildtype (WT) mice, indicating enhanced SERCA2a activity. In vitro and in vivo studies show that ALN is post-translationally modified via phosphorylation on Serine 19 (S19), suggesting this contributes to its ability to regulate SERCA2a. Immunoprecipitation and FRET analysis of ALN-WT, phospho-deficient ALN (S19A), or phosphomimetic ALN (S19D) revealed that S19 phosphorylation alters the SERCA2a-ALN interaction, leading to relief of its inhibitory effects. Adeno-associated virus mediated delivery of ALN-WT or phospho-mutant ALN-S19A/D in ALN KO mice showed that cardiomyocyte-specific expression of phospho-deficient ALN-S19A resulted in increased SERCA2a inhibition characterized by reduced rates of cytoplasmic Ca2+ clearance compared to ALN-WT and ALN-S19D expressing cells, further supporting a role for this phosphorylation event in controlling SERCA2a-regulation by ALN. Levels of ALN phosphorylation were markedly increased in cardiomyocytes in response to Gαq agonists (angiotensin II, endothelin-1, phenylephrine) and Gαq-mediated phosphorylation of ALN translated to increased Ca2+ cycling in cardiomyocytes from WT but not ALN KO mice. Collectively, these results indicate that ALN uniquely regulates Ca2+ handling in cardiomyocytes via integration of neuroendocrine signaling with SERCA2a activity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.70
自引率
0.00%
发文量
171
审稿时长
42 days
期刊介绍:
The Journal of Molecular and Cellular Cardiology publishes work advancing knowledge of the mechanisms responsible for both normal and diseased cardiovascular function. To this end papers are published in all relevant areas. These include (but are not limited to): structural biology; genetics; proteomics; morphology; stem cells; molecular biology; metabolism; biophysics; bioengineering; computational modeling and systems analysis; electrophysiology; pharmacology and physiology. Papers are encouraged with both basic and translational approaches. The journal is directed not only to basic scientists but also to clinical cardiologists who wish to follow the rapidly advancing frontiers of basic knowledge of the heart and circulation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


