包裹米托蒽醌和塞拉司琼的CaCO3络合pH响应纳米颗粒可增强肿瘤化疗免疫疗法。
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
在增强抗肿瘤免疫反应的同时调节免疫抑制性肿瘤微环境(TME)是一种前景广阔的策略。在这项研究中,我们设计了一种对酸敏感的纳米系统(MCCaNPs),通过全身性递送免疫刺激化疗组合物来展示有效的抗癌免疫疗法。我们采用双乳液法制备了含有 CaCO3 的 pH 响应纳米平台,并同时封装了米托蒽醌(MIT)和西拉斯特醇(CEL)作为免疫细胞死亡(ICD)诱导剂。由于 CaCO3 的酸响应性,纳米颗粒迅速消耗 H+ 以缓解酸性肿瘤微环境,并爆炸性地释放 CEL 和 MIT,在协同肿瘤化学免疫疗法中显示出固有的免疫调节活性。MIT 和 CEL 通过诱导肿瘤细胞释放钙粘蛋白(CRT)、高迁移率组盒 1 蛋白(HMGB1),协同触发更强的 ICD。静脉注射MCCaNPs后,小鼠肿瘤的局部肿瘤微环境(TME)发生了重编程。这种重编程的特点是肿瘤浸润细胞毒性T淋巴细胞(CTL)的密度显著增加,最终延长了生存期。因此,这项研究提出了一种很有前景的方法,即协同触发免疫原性细胞死亡,以提高肿瘤细胞毒性T淋巴细胞的浸润,从而达到抗癌免疫疗法的目的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
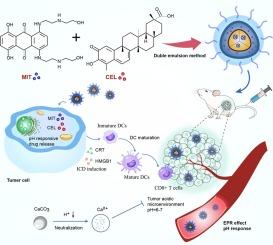
CaCO3-complexed pH-responsive nanoparticles encapsulating mitoxantrone and celastrol enhance tumor chemoimmunotherapy
Modulating the immunosuppressive tumor microenvironment (TME) while enhancing antitumor immune responses is a promising strategy. In this study, we designed an acid-sensitive nanosystem (MCCaNPs) to demonstrate effective immunotherapy against cancer through the systemic delivery of immune-stimulating chemotherapy combinations. A pH-responsive nanoplatform containing CaCO3 was prepared by the double emulsion method, and mitoxantrone (MIT) and celastrol (CEL) were simultaneously encapsulated as immunogenic cell death (ICD) inducers. Due to the acid responsiveness of CaCO3, the nanoparticles rapidly consume H+ to relieve the acidic tumor microenvironment and explosively release CEL and MIT, showing inherent immunomodulatory activity in collaborative tumor chemoimmunotherapy. MIT and CEL synergistically trigger stronger ICD by inducing tumor cells to release calreticulin (CRT), high mobility group box 1 protein (HMGB1). Following the intravenous administration of MCCaNPs, the local tumor microenvironment(TME) was reprogrammed in mice-bearing tumors. This reprogramming was characterized by a significant increase in the density of tumor-infiltrating cytotoxic T lymphocytes(CTLs), ultimately prolonging survival. Therefore, this research proposes a promising approach to trigger immunogenic cell death collaboratively, aiming to boost the tumor CTLs infiltration for anticancer immunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


