槲皮素通过靶向 PPARγ/PGC-1α/NF-κB 轴抑制有丝分裂介导的细胞凋亡和炎症反应,从而改善急性肝衰竭。
IF 4.8
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
摘要
背景:线粒体功能障碍产生的活性氧(ROS)是引发急性肝衰竭(ALF)中细胞凋亡和炎症的关键因素。槲皮素(QUE)是一种抗氧化剂,因其对肝脏疾病的治疗效果而闻名。目前还没有关于 QUE 是否能调节肝细胞有丝分裂水平以抑制 ALF 的研究:本研究探讨 QUE 对 ALF 的保护作用,并阐明其中的机制:方法:使用 LPS 和 D-Galn 建立 ALF 和肝细胞炎症损伤模型。为了预测 QUE 治疗 ALF 的潜在靶点和机制,研究人员采用了转录组学、网络药理学、分子对接技术和 ChIP 等方法。通过CCK8、IHC、IF染色、TUNEL、RT-qPCR、TEM、Western印迹、ELISA和流式细胞术检测了与有丝分裂、细胞凋亡和信号通路相关的表达水平:结果:网络药理学和转录组学发现了QUE和ALF的共同靶点。富集分析表明,QUE的抗ALF靶点与线粒体和NF-κB相关通路密切相关。随后的实验表明,QUE预处理能明显减轻肝细胞活力的丧失,提高线粒体膜电位,激活有丝分裂,促进受损线粒体的清除,从而减少ROS的积累,显著减少细胞凋亡和炎症反应,降低ALT和AST水平,改善肝组织病理学。从机理上讲,分子对接、DARTS和CETSA分析证实,QUE直接与PPARγ分子结合,减少了与IκB的结合,明显抑制了NF-κB通路,从而发挥其保护作用:总之,我们的研究结果首次证明了《QUE》可通过调节 PPARγ/PGC-1α/NF-κB 轴促进有丝分裂,抑制线粒体功能障碍介导的细胞凋亡和炎症反应,从而改善急性肝衰竭,这为《QUE》治疗 ALF 的潜力提供了证据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
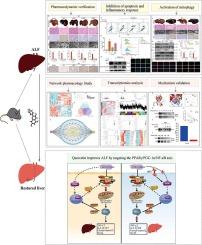
Quercetin inhibits mitophagy-mediated apoptosis and inflammatory response by targeting the PPARγ/PGC-1α/NF-κB axis to improve acute liver failure
Background
Reactive oxygen species (ROS) from mitochondrial dysfunction are critical in triggering apoptosis and inflammation in acute liver failure (ALF). Quercetin (QUE), an antioxidant, is renowned for its therapeutic effects on liver diseases. There are no studies on whether QUE regulates mitophagy level in hepatocytes to inhibit ALF.
Objective
This study investigates QUE’s protective effects on ALF and elucidates the mechanisms involved.
Methods
The ALF and hepatocyte inflammatory injury model was established using LPS and D-Galn. To predict potential targets and mechanisms of QUE in ALF treatment, transcriptomics, network pharmacology, molecular docking techniques, and ChIP were employed. The expression level related to mitophagy, apoptosis, and signaling pathways were detected by CCK8, IHC, IF staining, TUNEL, RT-qPCR, TEM, Western blotting, ELISA, and flow cytometry.
Results
Network pharmacology and transcriptomics revealed common targets between QUE and ALF. Enrichment analysis showed that the anti-ALF targets of QUE were significantly associated with mitochondria and NF-κB-related pathways. Subsequent experiments showed that QUE pretreatment significantly alleviated the loss of hepatocyte viability, enhanced mitochondrial membrane potential, activated mitophagy, and promoted the clearance of damaged mitochondria, thereby reducing ROS accumulation, significantly reducing cell apoptosis and inflammatory responses, reducing ALT and AST levels, and improving liver tissue pathology. Mechanistically, molecular docking, DARTS, and CETSA analyses confirmed that QUE directly binds to the PPARγ molecule, which reduced binding to IκB and significantly inhibit the NF-κB pathway to exert its protective effects.
Conclusion
In short, our results provide the first evidence that QUE improves acute liver failure by promoting mitophagy through regulating the PPARγ/PGC-1α/NF-κB axis and inhibiting apoptosis and inflammatory responses mediated by mitochondrial dysfunction, which provides evidence for the potential of QUE in the treatment of ALF.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


