通过分子动力学模拟探索三种β-内酰胺类抗生素与大肠杆菌青霉素结合蛋白之间的相互作用机制。
IF 3.9
3区 环境科学与生态学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Comparative Biochemistry and Physiology C-toxicology & Pharmacology
Pub Date : 2024-10-22
DOI:10.1016/j.cbpc.2024.110057
引用次数: 0
摘要
水生环境中存在的抗生素残留物对水生生物甚至人类健康都构成了巨大的潜在风险。阐明抗生素与生物大分子之间的相互作用机制对于准确评估和预防其潜在风险至关重要。因此,本研究采用时间依赖性毒性微孔板分析方法研究了三种β-内酰胺类抗生素对大肠杆菌(E. coli)的毒性。然后,利用分子对接和分子动力学模拟技术阐明了β-内酰胺类抗生素与大肠杆菌青霉素结合蛋白之间潜在的分子相互作用及其与生理生化实验中观察到的理化行为的相关性。结果表明,三种抗生素通过改变大肠杆菌细胞膜的通透性对其产生抑制作用,甚至会造成更严重的细胞损伤,包括破裂、皱缩、粘连、压痕、伸长和大小改变。但是,三种抗生素对大肠杆菌的毒性作用各不相同,毒性顺序依次为美罗培南 > 头孢哌酮 > 阿莫西林。范德华力在大肠杆菌的三种抗生素青霉素结合蛋白之间的分子相互作用中起着重要作用,结合自由能的顺序与观察到的毒性顺序一致。形状补偿是抗生素与青霉素结合蛋白结合的主要决定因素,它与药物引起的青霉素结合蛋白三维构象的改变有关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
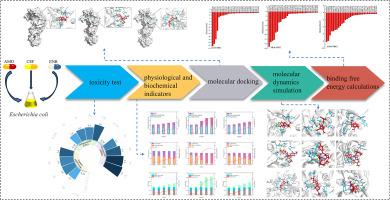
Probing the interaction mechanisms between three β-lactam antibiotics and penicillin-binding proteins of Escherichia coli by molecular dynamics simulations
The presence of antibiotic residues in the aquatic environments poses great potential risks to the aquatic organisms, and even human health. Elucidating the interaction mechanisms between antibiotics and biomacromolecules is crucial for accurately assessing and preventing their potential risks. Therefore, the toxicity of three beta-lactam antibiotics on Escherichia coli (E. coli) was investigated by using the time-dependent toxicity microplate analysis method in this study. Then, molecular docking and molecular dynamics simulation technologies were used to elucidate the potential molecular interactions between β-lactam antibiotics and penicillin-binding proteins of E. coli, and their correlation with the physical and chemical behaviors observed in the physiological and biochemical experiments. The results show that three antibiotics exert inhibitory effects on E. coli cells by modifying their membrane permeability, and even more severe cell damage including rupture, wrinkling, adhesion, indentation, elongation and size alterations. But, toxic effect of the three antibiotics on E. coli varies, and toxicity order is followed by meropenem > cefoperazone > amoxicillin. Van der Waals forces play a vital role in the molecular interactions between the three antibiotics penicillin binding protein of E. coli and the sequence of binding free energy is consistent with the observed toxicity order. Shape compensation is the principal determinant for the binding of antibiotics to penicillin binding proteins, which pertains to the drug-induced alteration in the three-dimensional conformation of penicillin binding proteins.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.50
自引率
5.10%
发文量
206
审稿时长
30 days
期刊介绍:
Part C: Toxicology and Pharmacology. This journal is concerned with chemical and drug action at different levels of organization, biotransformation of xenobiotics, mechanisms of toxicity, including reactive oxygen species and carcinogenesis, endocrine disruptors, natural products chemistry, and signal transduction with a molecular approach to these fields.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


