预测突变对蛋白质-蛋白质相互作用影响的图形掩蔽自馏学习。
IF 5.2
1区 生物学
Q1 BIOLOGY
引用次数: 0
摘要
评估突变对蛋白质-蛋白质相互作用(PPIs)的结合亲和力变化(ΔΔG)的影响在揭示蛋白质结构-功能的复杂性和开发创新蛋白质设计方面起着至关重要的作用。在本研究中,我们提出了一种深度学习框架 PIANO,用于改进 PPI 中 ΔΔG 的预测。PIANO框架利用图掩蔽自馏分方案进行蛋白质结构几何表征预训练,有效捕捉突变位点周围的结构上下文表征,并利用由氨基酸、原子和蛋白质序列的多个编码器组成的多分支网络进行预测。广泛的实验证明了其卓越的预测性能和预训练编码器捕捉有意义表征的能力。与之前的方法相比,PIANO 可广泛应用于全复合物结构和单体结构。此外,我们还展示了 PIANO 在突出致病突变和关键蛋白方面的实际应用能力,以及在 PPI 系统中区分疾病病例和对照组中的新发突变的能力。总之,PIANO 提供了一种强大的深度学习工具,可为药物设计、治疗干预和蛋白质工程研究提供有价值的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
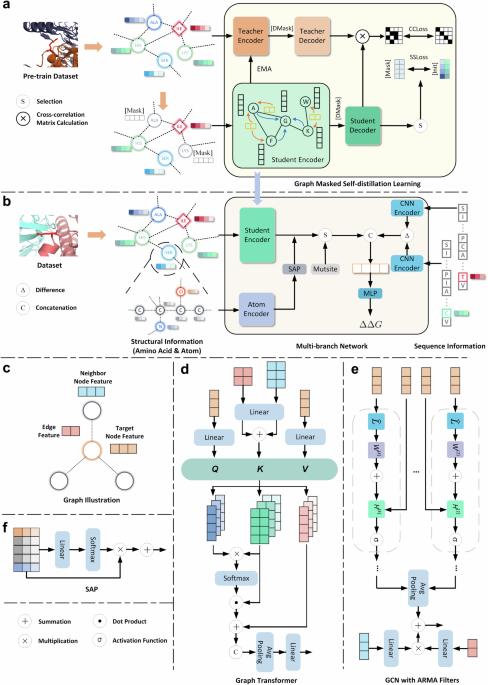
Graph masked self-distillation learning for prediction of mutation impact on protein–protein interactions
Assessing mutation impact on the binding affinity change (ΔΔG) of protein–protein interactions (PPIs) plays a crucial role in unraveling structural-functional intricacies of proteins and developing innovative protein designs. In this study, we present a deep learning framework, PIANO, for improved prediction of ΔΔG in PPIs. The PIANO framework leverages a graph masked self-distillation scheme for protein structural geometric representation pre-training, which effectively captures the structural context representations surrounding mutation sites, and makes predictions using a multi-branch network consisting of multiple encoders for amino acids, atoms, and protein sequences. Extensive experiments demonstrated its superior prediction performance and the capability of pre-trained encoder in capturing meaningful representations. Compared to previous methods, PIANO can be widely applied on both holo complex structures and apo monomer structures. Moreover, we illustrated the practical applicability of PIANO in highlighting pathogenic mutations and crucial proteins, and distinguishing de novo mutations in disease cases and controls in PPI systems. Overall, PIANO offers a powerful deep learning tool, which may provide valuable insights into the study of drug design, therapeutic intervention, and protein engineering. PIANO: a deep learning framework providing a powerful tool and potentially unforeseen avenues for the prediction of mutation impact on the binding affinity changes of protein–protein interactions
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Biology
Medicine-Medicine (miscellaneous)
CiteScore
8.60
自引率
1.70%
发文量
1233
审稿时长
13 weeks
期刊介绍:
Communications Biology is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the biological sciences. Research papers published by the journal represent significant advances bringing new biological insight to a specialized area of research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


