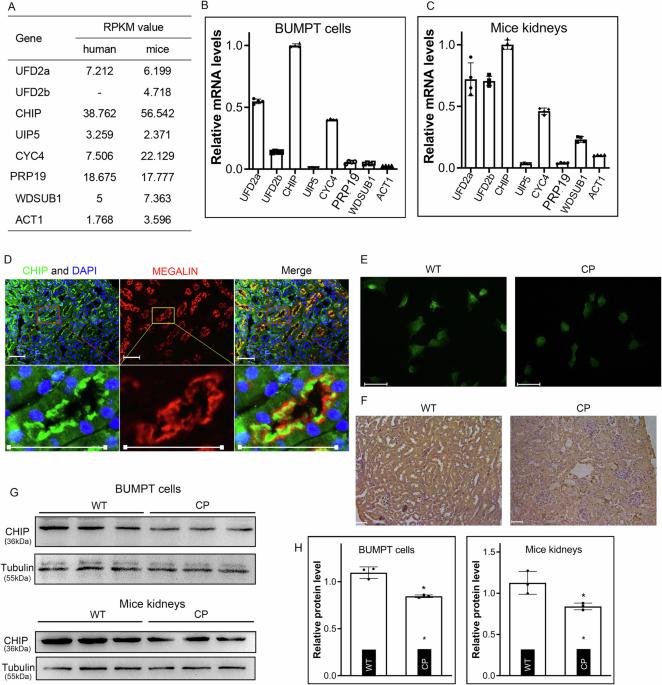
在顺铂诱导的肾病中,CHIP 驱动蛋白酶体降解 NUR77 以减轻氧化应激和内源性凋亡。
IF 5.2
1区 生物学
Q1 BIOLOGY
引用次数: 0
摘要
Hsc70相互作用蛋白的羧基末端(CHIP)是一种E3连接酶,它能调节目标蛋白的稳定性,从而缓解各器官系统的各种病理紊乱。顺铂是一种广泛使用的化疗药物,但其肾毒性也是众所周知的。CHIP在顺铂诱导的急性肾损伤(AKI)发病机制中的作用尚未得到充分研究。在本文中,我们证实 CHIP 在肾近曲小管上皮中大量表达,并且在顺铂诱导的 AKI 中表达下调。进一步研究发现,CHIP过表达或激活可减轻顺铂诱导的肾近曲小管上皮细胞氧化应激和细胞凋亡,而其基因破坏则可促进氧化应激和细胞凋亡。在机制方面,CHIP 与 NUR77 相互作用并泛素化,促进其降解,从而保护 BCL2,在顺铂存在的情况下维持肾近曲小管细胞线粒体的通透性。此外,我们还证明了 CHIP 通过其中央盘卷(CC)结构域与 NUR77 相互作用,这是一种非经典的相互作用模式。总之,这些研究结果表明,CHIP泛素化并降解其底物NUR77,从而减轻了顺铂处理的肾近曲小管上皮细胞的内在凋亡,从而为顺铂诱导的AKI的发病机制提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
CHIP drives proteasomal degradation of NUR77 to alleviate oxidative stress and intrinsic apoptosis in cisplatin-induced nephropathy
Carboxy-terminus of Hsc70-interacting protein (CHIP), an E3 ligase, modulates the stability of its targeted proteins to alleviate various pathological perturbations in various organ systems. Cisplatin is a widely used chemotherapeutic agent, but it is also known for its alarming renal toxicity. The role of CHIP in the pathogenesis of cisplatin-induced acute kidney injury (AKI) has not been adequately investigated. Herein, we demonstrated that CHIP was abundantly expressed in the renal proximal tubular epithelia, and its expression was downregulated in cisplatin-induced AKI. Further investigation revealed that CHIP overexpression or activation alleviated, while its gene disruption promoted, oxidative stress and apoptosis in renal proximal tubular epithelia induced by cisplatin. In terms of mechanism, CHIP interacted with and ubiquitinated NUR77 to promote its degradation, which consequently shielded BCL2 to maintain mitochondrial permeability of renal proximal tubular cells in the presence of cisplatin. Also, we demonstrated that CHIP interacted with NUR77 via its central coiled-coil (CC) domain, a non-canonical interactive pattern. In conclusion, these findings indicated that CHIP ubiquitinated and degraded its substrate NUR77 to attenuate intrinsic apoptosis in cisplatin-treated renal proximal tubular epithelia, thus providing a novel insight for the pathogenesis of cisplatin-induced AKI. CHIP interacts with NUR77 via its coiled-coil domain in renal proximal tubular cells, and their interaction leads to the K48 ubiquitination of NUR77 and its subsequent proteasomal degradation, resulting in the alleviation of cisplatin-induced AKI.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Biology
Medicine-Medicine (miscellaneous)
CiteScore
8.60
自引率
1.70%
发文量
1233
审稿时长
13 weeks
期刊介绍:
Communications Biology is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the biological sciences. Research papers published by the journal represent significant advances bringing new biological insight to a specialized area of research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


