核苷酸介导的液态凝聚相中化学选择性蛋白质功能化的调制。
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
液态蛋白质凝聚物在细胞系统中无处不在,其在生理过程中的作用也日益得到认可。凝聚相具有独特的化学微环境,与稀释的水相明显不同。在此,我们展示了蛋白质的亲核氨基酸侧链(即半胱氨酸、酪氨酸和赖氨酸)在稀释相和凝聚相中对 4-Cloro-7-nitrobenzofurazan 的化学选择性修饰模式。我们还描述了核苷酸的作用及其原位酶解如何在时间上调节蛋白质凝聚物的 pH 值以及蛋白质相应的化学选择性修饰。我们已经证明,在存在三磷酸核苷的情况下,缩合物的 pH 值会降低,而在存在单磷酸核苷或磷酸离子的情况下,pH 值会升高。例如,我们发现赖氨酸特异性修饰在有三磷酸腺苷(ATP)存在时会受到抑制,但在有单磷酸盐存在时会显著增强。这一特点使我们能够通过酶促 ATP 水解对蛋白质功能化的动态变化进行时间控制。总之,这项研究证实了蛋白质的ε-胺在凝聚相中对 pH 值的反应性改变。此外,凝结相中化学选择性蛋白质官能化的这种环境敏感性,对于适应蛋白质相分离的化学生物学的蛋白质工程学非常重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
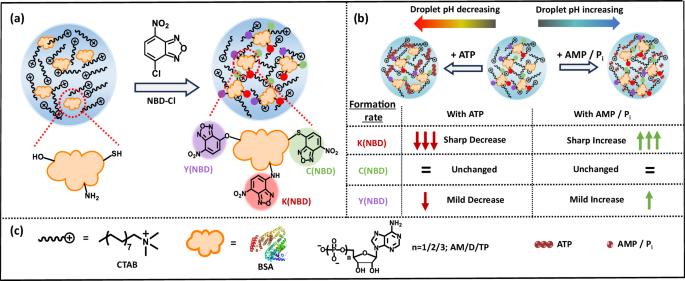
Nucleotide-mediated modulation of chemoselective protein functionalization in a liquid-like condensed phase
Liquid-like protein condensates are ubiquitous in cellular system and are increasingly recognized for their roles in physiological processes. Condensed phase harbors distinctive chemical microenvironment, markedly different than dilute aqueous phase. Herein, we demonstrate chemoselective modification pattern of nucleophilic canonical amino acid sidechains (namely – cysteine, tyrosine and lysine) of the protein towards 4-chloro-7-nitrobenzofurazan in the dilute and condensed phase. We also delineate how the effect of nucleotides and their in situ enzymatic dissociation temporally modulate the protein condensate’s pH and the protein’s corresponding chemoselective modification. We have shown that the pH of the condensate decreases in the presence of nucleoside triphosphate, whereas it increases in the presence of nucleoside monophosphates or phosphate ion. For instance, we find lysine-specific modification gets inhibited in the presence of adenosine triphosphate (ATP), but significantly enhanced in the presence of monophosphates. This feature enables us to gain temporal control over dynamic change in protein functionalization via enzymatic ATP hydrolysis. Overall, this work substantiates the alteration in pH-responsiveness of Brønsted basicity of a protein’s ε-amine in the condensed phase. Furthermore, this environment sensitivity in chemoselective protein functionalization in condensed phase will be important in adaptable protein engineering to the chemical biology of protein phase separation. Liquid-like protein condensates are ubiquitous in cellular systems and play key roles in physiological processes, with the condensed phase harboring a distinctive chemical microenvironment. Here, the authors systematically investigate how the local chemical environment within protein condensates impacts chemical reactivity, demonstrating nucleotide-mediated modulation of chemoselective protein functionalization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


