橙皮甙通过AMPKα-Drp1/PINK1-Parkin信号通路调节线粒体动力学和有丝分裂,从而预防非酒精性脂肪性肝炎。
IF 3.9
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular and cell biology of lipids
Pub Date : 2024-10-23
DOI:10.1016/j.bbalip.2024.159570
引用次数: 0
摘要
非酒精性脂肪肝(NAFLD)正成为全球公共卫生的一大负担,但目前却明显缺乏有效的治疗策略。新研究发现,非酒精性脂肪肝的发生可能是由线粒体功能障碍介导的。从植物性物质中提取线粒体调节剂来治疗线粒体功能障碍是治疗非酒精性脂肪肝的一种有吸引力的方法。橙皮素(HES)是一种天然黄酮类化合物,属于黄烷酮家族。本研究旨在阐明橙皮甙预防高脂饮食(HFD)引起的非酒精性脂肪肝的机制。研究人员对高脂饮食诱发的非酒精性脂肪肝斯普拉格-道利(SD)大鼠的血清和肝脏生化指标、肝脏组织学、血脂谱和线粒体功能进行了评估。HES 治疗能明显降低非酒精性脂肪肝大鼠的体重增加、肝脏重量和肝脏指数,同时还能改善非酒精性脂肪肝大鼠的肝脏脂肪变性、脂质代谢紊乱和线粒体功能障碍。研究人员利用 Western 印迹和实时定量聚合酶链式反应(RT-qPCR)对其机制进行了研究和证实。我们发现,在非酒精性脂肪肝大鼠的肝脏中,HES通过AMP激活的蛋白激酶α(AMPKα)依赖机制降低了动态相关蛋白1(Drp1)、丝氨酸-616处磷酸化的Drp1(Drp1-pS616)的表达,并诱导了丝氨酸-637处磷酸化的Drp1(Drp1-pS637)、PTEN诱导的激酶1(PINK1)和E3泛素蛋白连接酶Parkin(Parkin)的表达。此外,HES 增加了线粒体融合蛋白 mitofusin-2 (Mfn2) 和视神经萎缩 1 (Opa1) 的表达,同时抑制了裂变蛋白 1 (Fis1) 的表达。在这项研究中,我们发现了 HES 防止非酒精性脂肪肝发生的独特机制。HES可能是一种具有吸引力的治疗非酒精性脂肪肝的潜在药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
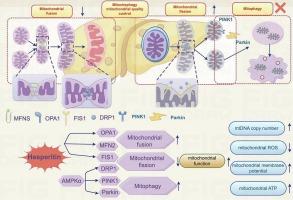
Hesperitin prevents non-alcoholic steatohepatitis by modulating mitochondrial dynamics and mitophagy via the AMPKα-Drp1/PINK1-Parkin signaling pathway
Non-alcoholic fatty liver disease (NAFLD) is becoming a global public health burden, yet effective therapeutic strategies are notably lacking. NAFLD development may be mediated by mitochondrial dysfunction, according to new research. Producing mitochondrial regulators from plant-based substances to treat mitochondrial dysfunction is an appealing approach to treating NAFLD. Hesperetin (HES) is a flavonoid that is found naturally and is a member of the flavanone family. This study aims to clarify the mechanism of HES in preventing NAFLD which is caused by a high-fat diet (HFD). Serum and liver biochemical parameters, liver histology, lipid profiles, and mitochondrial function were evaluated in HFD-induced NAFLD Sprague-Dawley (SD) rats. HES treatment significantly reduced body weight gain, liver weight, and the liver index, while also improving hepatic steatosis, lipid metabolism disorders, and mitochondrial dysfunction in rats with NAFLD. The mechanism was investigated and confirmed using western blot and real-time quantitative polymerase chain reaction (RT-qPCR). We showed that in the liver of NAFLD rats, HES decreased the expression of dynamic-related protein 1 (Drp1), phosphorylated Drp1 at serine-616 (Drp1-pS616) and induced phosphorylated Drp1 at serine-637 (Drp1-pS637), PTEN-induced kinase 1 (PINK1), and E3 Ubiquitin-Protein Ligase Parkin (Parkin) via an AMP-activated protein kinase alpha (AMPKα)-dependent mechanism. Moreover, HES increased the expression of the mitochondrial fusion proteins mitofusin-2 (Mfn2) and optic atrophy 1 (Opa1) while suppressing the expression of fission protein 1 (Fis1). In this work, we identify a unique mechanism by which HES prevents NAFLD from developing. HES may be an attractive potential therapeutic agent to cure NAFLD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.00
自引率
2.10%
发文量
109
审稿时长
53 days
期刊介绍:
BBA Molecular and Cell Biology of Lipids publishes papers on original research dealing with novel aspects of molecular genetics related to the lipidome, the biosynthesis of lipids, the role of lipids in cells and whole organisms, the regulation of lipid metabolism and function, and lipidomics in all organisms. Manuscripts should significantly advance the understanding of the molecular mechanisms underlying biological processes in which lipids are involved. Papers detailing novel methodology must report significant biochemical, molecular, or functional insight in the area of lipids.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


