原子力显微镜揭示的染色质凝聚结构域及其在机械变形的正常细胞核和恶性细胞核中的转变。
IF 2.5
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochemical and biophysical research communications
Pub Date : 2024-10-23
DOI:10.1016/j.bbrc.2024.150861
引用次数: 0
摘要
人们普遍认为,异染色质是一种规则、致密和封闭的结构,而常染色质则是开放和稀疏的。最近的证据表明,染色质是由不规则的核小体簇组成的,它们在细胞核内紧密结合。转录事件改变了染色质结构,导致出现 100-300 nm 的核小体聚集体。与此同时,目前的染色质结构范式在很大程度上是支离破碎的。在这篇通讯中,我们通过对细胞核进行机械变形和原子力显微镜(AFM)分析,揭示了正常细胞核和恶性细胞核的染色质超微结构。在人类皮肤成纤维细胞核中,我们发现了约 16.5-33.5 纳米的纳米域。观察到正常细胞核染色质的分层折叠:纳米域形成不规则的纤维状结构,并凝聚成染色质大区。在纤维肉瘤细胞核中,发现了约 66.3-113.0 nm 的 DNA 超卷曲域(SD),它们均匀地分布在细胞核内。通过上调和下调超卷曲,染色质凝聚域的形态发生了变化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
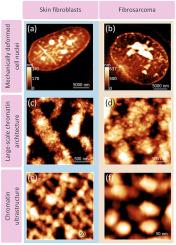
Chromatin condensed domains revealed by AFM, and their transformation in mechanically deformed normal and malignant cell nuclei
It has been generally accepted that heterochromatin is represented by a regular, dense and closed structure, while euchromatin is open and sparse. Recent evidence indicates that chromatin is comprised of irregular nucleosome clutches compacted within the nucleus. Transcriptional events transform the chromatin architecture, resulting in appearance of 100–300 nm nucleosomal aggregates. Meanwhile, the current paradigm of chromatin architecture is largely fragmented. In this communication, we unraveled chromatin ultrastructure of normal and malignant cell nuclei through mechanical deformation of the nuclei and Atomic Force Microscopy (AFM) analysis of the resulting landscape. In human skin fibroblasts cell nuclei, nanodomains of about 16.5–33.5 nm were revealed. Hierarchical folding of the chromatin of normal nuclei was observed: the nanodomains formed irregular fiber-like structures that coalesced into the macroscale chromatin compartments. In fibrosarcoma cell nuclei DNA supercoiling domains (SDs) of about 66.3–113.0 nm, uniformly distributed within the nuclei, were revealed. Transformation of the morphology of the condensed chromatin domains through up- and downregulation of supercoiling was demonstrated.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.10
自引率
0.00%
发文量
1400
审稿时长
14 days
期刊介绍:
Biochemical and Biophysical Research Communications is the premier international journal devoted to the very rapid dissemination of timely and significant experimental results in diverse fields of biological research. The development of the "Breakthroughs and Views" section brings the minireview format to the journal, and issues often contain collections of special interest manuscripts. BBRC is published weekly (52 issues/year).Research Areas now include: Biochemistry; biophysics; cell biology; developmental biology; immunology
; molecular biology; neurobiology; plant biology and proteomics

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


