基于生物素蛋白连接酶反应的 ATP 检测系统。
IF 2.5
4区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
三磷酸腺苷(ATP)是所有生物的能量货币,可用作细胞增殖和细胞毒性的指标。在本研究中,我们开发了一种新型的 ATP 检测系统,该系统将托克代磺酸古菌的生物素化反应与荧光共振能量转移(FRET)相结合。在托克达伊硫球菌的生物素化反应中,一种被称为生物素蛋白连接酶(BPL)的酶与其产物--生物素化底物蛋白(BCP)--形成非常稳定的复合物。在这里,BPL 和 BCCP 分别与荧光蛋白 Cerulean 和 Clover 融合,并通过监测两种荧光蛋白之间的 FRET 信号来实现 ATP 检测,因为 ATP 是生物素化的必要成分,而且只有在生物素化之后才会形成紧密的 BPL-BCCP 复合物。利用这一系统,我们成功地通过生物素化反应检测到了 5 nM 的 ATP 与 50 nM 的融合蛋白。我们的方法有一个特点,即信号在反应开始后至少 2 小时内不会衰减,这与常用于 ATP 检测的荧光素酶发光法不同。因此,我们的系统在进行 ATP 检测时不会受到测量时间的明显限制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
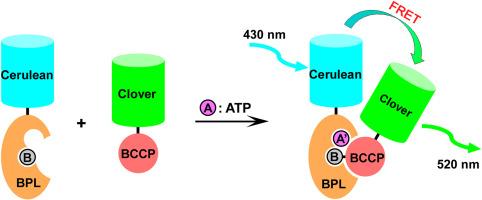
An ATP detection system based on the enzyme reaction with biotin protein ligase
Adenosine triphosphate (ATP) is the energy currency of all living organisms and can be used as an indicator for cell proliferation and cytotoxicity. In the present work, we have developed a novel ATP detection system by combining the biotinylation reaction from archaeon Sulfolobus tokodaii with fluorescence resonance energy transfer (FRET). In biotinylation from S. tokodaii, an enzyme known as biotin protein ligase (BPL) forms a very stable complex with its product, biotinylated substrate protein (BCCP). Here, BPL and BCCP were fused to the fluorescent proteins Cerulean and Clover, respectively, and ATP detection was accomplished by monitoring the FRET signal between the two fluorescent proteins, since ATP is an essential component for biotinylation and the tight BPL-BCCP complex is formed only after biotinylation. Using this system, we have succeeded in detecting 5 nM of ATP by biotinylation reaction with 50 nM of each fusion protein. Our method has a characteristic that the signal does not decay for at least 2 h after the start of the reaction, unlike in the case of the luminescence-based assay with luciferase commonly used for the ATP detection. Thus, our system allows for ATP detection which is not significantly constrained by measurement timing.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical biochemistry
生物-分析化学
CiteScore
5.70
自引率
0.00%
发文量
283
审稿时长
44 days
期刊介绍:
The journal''s title Analytical Biochemistry: Methods in the Biological Sciences declares its broad scope: methods for the basic biological sciences that include biochemistry, molecular genetics, cell biology, proteomics, immunology, bioinformatics and wherever the frontiers of research take the field.
The emphasis is on methods from the strictly analytical to the more preparative that would include novel approaches to protein purification as well as improvements in cell and organ culture. The actual techniques are equally inclusive ranging from aptamers to zymology.
The journal has been particularly active in:
-Analytical techniques for biological molecules-
Aptamer selection and utilization-
Biosensors-
Chromatography-
Cloning, sequencing and mutagenesis-
Electrochemical methods-
Electrophoresis-
Enzyme characterization methods-
Immunological approaches-
Mass spectrometry of proteins and nucleic acids-
Metabolomics-
Nano level techniques-
Optical spectroscopy in all its forms.
The journal is reluctant to include most drug and strictly clinical studies as there are more suitable publication platforms for these types of papers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


