一种茄科甾体生物碱毒素在菜青虫(Trichoplusia ni)体内的命运。
IF 3.2
2区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
植物对食草动物产生复杂的化学防御,导致植食性昆虫出现解毒策略。虽然酶解毒和靶点诱变都有详细记载,但对排泄的定量贡献研究仍然较少。我们以卷心菜卷叶蛾(Trichoplusia ni)这种通食性食草动物为研究对象,阐明了马铃薯(Solanum tuberosum)中产生的甾体生物碱茄尼丁的解毒过程。通过幼虫喂养实验和使用高分辨率质谱对代谢物进行化学分析,我们确定茄尼啶 3-O-β- 吡喃葡萄糖苷和茄尼啶 3-磷酸是茄尼啶的主要代谢产物。糖基化和磷酸化反应以前从未在菜青虫中观察到。改性茄尼啶衍生物的亲脂性降低,无法像理化分析预测的那样进行被动运输,在身体组织中只检测到茄尼啶。此外,还研究了中肠粘连蛋白被敲除的 T. ni 突变株的茄尼啶代谢情况,以考察粘连蛋白(Bt 毒素的重要受体)在甾体生物碱解毒过程中的潜在作用。与野生型菌株(51.3% ± 4.1, 33.66 ± 2.48 nmol/g)相比,T. ni 粘连蛋白敲除菌株的茄尼啶转化率(33.9% ± 2.2)和吸收率(27.41 ± 0.49 nmol/g)较低,但排泄动力学相似。虽然索拉尼定对两个品系的摄食性能都有负面影响,但卡德林基因敲除并不影响摄食性能。我们的研究扩大了卷心菜环斑蝶类代谢酶的范围,强调了通食性食草动物解毒机制的复杂性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
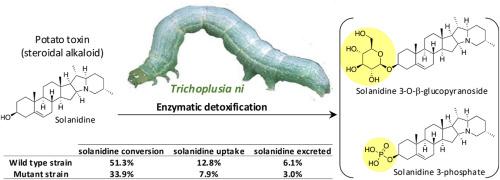
The fate of a Solanum steroidal alkaloid toxin in the cabbage looper (Trichoplusia ni)
Plants produce complex chemical defenses against herbivores, resulting in the emergence of detoxification strategies in phytophagous insects. While enzymatic detoxification and target site mutagenesis are well-documented, the quantitative contribution of excretion remains less studied. We focus on the cabbage looper (Trichoplusia ni), a generalist herbivore, to elucidate the detoxification of a steroidal alkaloid, solanidine, produced in potato (Solanum tuberosum). Through larval feeding experiments and chemical analysis of metabolites using high-resolution mass spectrometry, we identify solanidine 3-O-β-glucopyranoside and solanidine 3-phosphate as major metabolization products of solanidine. Glycosylation and phosphorylation reactions have not previously been observed in cabbage looper. Modified solanidine derivatives exhibit reduced lipophilicity, preventing passive transport as predicted by physicochemical analyses, and only solanidine was detected in body tissue. In addition, the metabolism of solanidine in a T. ni mutant strain with midgut cadherin protein knocked out was also investigated to examine the potential role of the cadherin, an important receptor for Bt toxins, in steroidal alkaloid detoxification. T. ni cadherin-knockout strain showed lower solanidine conversion (33.9% ± 2.2) and uptake (27.41 ± 0.49 nmol/g) compared to the wild-type strain (51.3% ± 4.1, 33.66 ± 2.48 nmol/g) but similar excretion kinetics. Although solanidine negatively impacted the feeding performance of both strains the cadherin-knockout does not affect the feeding performance. Our study expands the metabolization enzyme repertoire in cabbage loopers, emphasizing the complexity of detoxification mechanisms in generalist herbivores.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.40
自引率
5.30%
发文量
105
审稿时长
40 days
期刊介绍:
This international journal publishes original contributions and mini-reviews in the fields of insect biochemistry and insect molecular biology. Main areas of interest are neurochemistry, hormone and pheromone biochemistry, enzymes and metabolism, hormone action and gene regulation, gene characterization and structure, pharmacology, immunology and cell and tissue culture. Papers on the biochemistry and molecular biology of other groups of arthropods are published if of general interest to the readership. Technique papers will be considered for publication if they significantly advance the field of insect biochemistry and molecular biology in the opinion of the Editors and Editorial Board.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


