利用放射性223Ra/Ba SAzymes靶向衰老,实现衰老分解-解锁-一举两得战略,促进抗肿瘤免疫疗法。
IF 12.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
衰老细胞的特点是细胞周期持续停止,这使它们成为癌症治疗中抗肿瘤策略的重要目标。许多研究都将诱导衰老作为一种有前景的肿瘤治疗方法。然而,这些治疗方法往往存在缺点,包括不良副作用和较弱的衰老诱导效应。为了应对这些挑战,我们合成了223Ra/Ba单原子纳米酶(SAzyme),其中Ba SAzyme同时作为223RaCl2的载体,促进靶向递送并最大限度地减少副作用。223Ra/Ba SAzyme 复合物可增强各种酶模拟功能,包括过氧化氢酶(CAT)和过氧化物酶(POD)活性。重要的是,223Ra/Ba SAzyme 能诱导细胞衰老,增强抗肿瘤免疫力。衰老相关分泌表型(SASP)在肿瘤微环境中的持续存在会带来免疫抑制和肿瘤复发的风险,而衰老解毒剂可以有效缓解这种风险。因此,223Ra/Ba SAzyme与抗PD-L1检查点阻断相结合,实现了一举两得的疗法,即223Ra/Ba SAzyme利用衰老,然后通过抗PD-L1疗法消灭衰老细胞。这种一举两得的策略方法为原发性肿瘤和远处肿瘤提供了一种直接而有效的干预手段。本文章由计算机程序翻译,如有差异,请以英文原文为准。
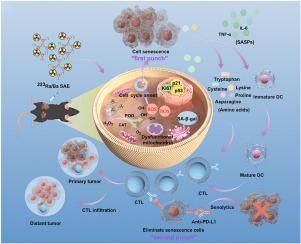
Targeting senescence with radioactive 223Ra/Ba SAzymes enables senolytics-unlocked One‐Two punch strategy to boost anti-tumor immunotherapy
Senescent cells are characterized by a persistent cessation of their cell cycle, rendering them valuable targets for anti-tumor strategies in cancer treatment. Numerous studies have explored induced senescence as a promising approach in tumor therapy. Nevertheless, these treatments often come with drawbacks, including adverse side effects and weaker senescence-inducing effects. To address these challenges, we synthesized 223Ra/Ba single-atom nanozyme (SAzyme), wherein Ba SAzyme acts concurrently as a carrier for 223RaCl2, facilitating targeted delivery and minimizing side effects. The 223Ra/Ba SAzyme complex enhances various enzyme-mimicking functions, including catalase (CAT) and peroxidase (POD) activities. Importantly, 223Ra/Ba SAzyme induces cellular senescence and boost anti-tumor immunity. The persistent presence of a senescence-associated secretory phenotype (SASP) in the tumor microenvironment presents risks of immune suppression and tumor recurrence, which can be effectively mitigated by senolytics. As a result, 223Ra/Ba SAzyme were combined with anti-PD-L1 checkpoint blockade to achieve a one-two punch therapy, wherein 223Ra/Ba SAzyme exploits senescence followed by anti-PD-L1 therapy to eradicate senescent cells. This one‐two punch strategy approach presents a straightforward and potent intervention for both primary tumors and distant tumor.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


