一种基于硫醇-色素点击反应的线粒体靶向比色和近红外比色荧光探针,用于检测具有较大斯托克斯位移的生物硫醇。
IF 2.9
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本研究巧妙地设计和合成了一种基于咔唑的线粒体靶向比色和近红外比色荧光探针 1,该探针是基于硫醇-色素点击反应的生物硫醇探针。在与生物硫醇(Cys、Hcy 和 GSH)相互作用时,1 的吸收从 496 纳米转移到 388 纳米,而其荧光光谱则从 650 纳米转移到 530 纳米。这些转变伴随着可见光下从粉红色到无色,以及在 365 纳米紫外灯下从红色到绿色的可见颜色变化,其原因可能是生物硫醇与铬烯分子中的α,β-不饱和酮发生了点击反应,随后吡喃开环和苯酚形成,以及对羟基苄基发生 1,6 消去反应,生成了 2。1 的这些进步使我们能够以高灵敏度(Cys、GSH 和 Hcy 的 LOD 分别为 97 nM、94 nM 和 93 nM)、大斯托克斯位移(154 nm)和出色的选择性按比例检测生物硫醇。此外,1 还可以靶向线粒体,并通过荧光比率测量法对细胞内生物硫醇的波动进行成像。此外,还首次提出了在喹啉的 N 原子上修饰色烯的新颖设计策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
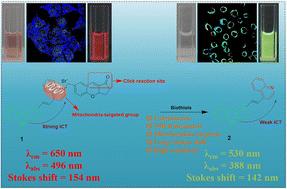
A mitochondria-targeted colorimetric and NIR ratiometric fluorescent probe for biothiols with large Stokes shift based on thiol–chromene click reaction†
In this study, a carbazole-based mitochondria-targeted colorimetric and NIR ratiometric fluorescent probe for biothiols based on the thiol–chromene click reaction was subtly designed and synthesized. Upon interaction with biothiols (Cys, Hcy and GSH), the absorption of shifted from 496 nm to 388 nm, while its fluorescence spectrum shifted from 650 nm to 530 nm. These transformations were accompanied by a visible color change from pink to colorless under visible light and from red to green when observed under a 365 nm UV lamp, which can be attributed to the click reaction of biothiols with the α,β-unsaturated ketone of the chromene moiety, subsequent pyran ring-opening and phenol formation as well as 1,6-elimination of a p-hydroxybenzyl moiety yielding . These advancements in have allowed us to ratiometrically detect biothiols with high sensitivity (LODs of 97 nM, 94 nM and 93 nM for Cys, GSH and Hcy, respectively), a large Stokes shift (154 nm) and excellent selectivity. In addition, can target mitochondria and image the fluctuation of intracellular biothiols through fluorescence ratiometry. Furthermore, the novel design strategy of modifying chromene to the N atom of quinoline was proposed for the first time.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


