受四键相互作用的影响,脯氨酰氨基甲酸酯中的顺式PR稳定。
IF 2.9
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
对同源脯氨酰氨基甲酸酯的核磁共振光谱和理论分析揭示了微妙的电荷转移四键相互作用(TBI),选择性地稳定了它们的顺式 Pro 转子。这些 TBI 涉及 C 端酰胺到 N 端氨基甲酸酯羰基-羰基(n → π* 型),然后是氨基甲酸酯内部(n → σ* 型)的电荷转移相互作用,这些相互作用完全发生在 cisPro 主题中。TBI 的数量以及 cisPro 的稳定性随着氨基甲酸乙酯上 Cβ 基团数量的增加而增加。溶剂极性的增加也会提高顺式 Pro 氨基甲酸酯的相对稳定性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
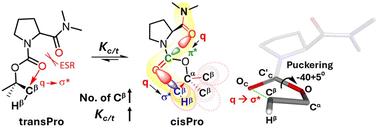
cisPro stabilization in prolyl carbamates influenced by tetrel bonding interactions†
NMR spectral and theoretical analyses of homologous prolyl carbamates reveal subtle charge transfer tetrel bonding interactions (TBIs), selectively stabilizing their cisPro rotamers. These TBIs involve C-terminal-amide to N-terminal carbamate carbonyl–carbonyl (n → π* type) followed by intra-carbamate (n → σ* type) charge transfer interactions exclusively in the cisPro motif. The number of TBIs and hence the cisPro stability increase with increasing number of Cβ groups at the carbamate alcohol. Increasing solvent polarities also increase the relative cisPro carbamate stabilities.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


