α-官能化烷基碘化物与炔烃的立体异构原子转移自由基加成:一种选择性合成 E- 和 Z-iodo 烷烯的策略。
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
炔烃原子转移自由基加成(ATRA)反应的几何控制是一项重大挑战。在此,我们提出了一种统一的解决方案,即从相同的材料出发,开发出一种立体异构合成方法,可同时合成所得烯产物的两种异构体。热力学上更稳定的异构体的合成采用了上坡催化策略,而稳定性较差的异构体的积累则是通过锰催化的碘萃取/自由基反弹过程,利用其可逆性来实现的。利用各种取代的烷基碘化物,可以很容易地获得在烯丙基位置上带有 CF3、CF2H、CN、酯或酰胺等有价值官能团的碘烷烃产品的两种异构体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
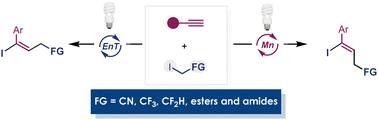
Stereodivergent atom transfer radical addition of α-functionalized alkyl iodides to alkynes: a strategy for selective synthesis of both E- and Z-iodoalkenes†
The geometrical control of atom transfer radical addition (ATRA) reactions to alkynes poses significant challenges. Herein, we present a uniform solution by developing a stereodivergent synthetic method for both isomers of the resulting alkene products, starting from the same materials. The synthesis of the thermodynamically more stable isomer utilizes the strategy of uphill catalysis while the accumulation of the less stable isomer is facilitated by a manganese-catalyzed iodo-abstraction/radical rebound process, taking advantage of its reversibility. Various substituted alkyl iodides can be used to provide easy access to both isomers of iodoalkene products with valuable functional groups such as CF3, CF2H, CN, ester, or amide at the allylic position.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


