通过 Al3+ 掺杂和 LiAlO2 涂层提高 LiNiO2 的循环稳定性
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在这项研究中,我们解决了 LiNiO2 循环和速率性能差的问题。LiNiO2 是一种镍含量超高的材料,被认为是高能量密度锂离子电池正极的有力竞争者。我们在锂化过程中引入了纳米 Al2O3,通过体相元素掺杂和原位 LiAlO2 涂层实现了材料的双重改性。比较结果表明,改性材料的性能显著提高。其中,在 0.5C 电流密度下进行的长周期测试中,LiNi0.99Al0.01O2 在 300 个周期后的容量保持率达到 73.1%,优于未掺杂的材料。在速率性能测试中,掺杂样品的放电特定容量始终高于未掺杂样品。值得注意的是,在 5C 的高电流密度下,LiNi0.99Al0.01O2 的放电特定容量为 101.75 mAh g-1。结果表明,适量的铝掺杂能有效稳定阴极材料的层状结构,并延迟从 H2 到 H3 的不可逆相变。此外,铝掺杂还有助于在颗粒表面形成一层 LiAlO2 涂层。这种涂层可作为快速离子导体,增强锂离子的传输,减少电解质对活性材料的侵蚀。本文章由计算机程序翻译,如有差异,请以英文原文为准。
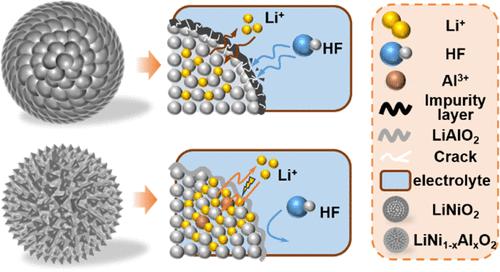
Improving the Cycle Stability of LiNiO2 through Al3+ Doping and LiAlO2 Coating
In this study, we addressed the poor cycling and rate performance of LiNiO2, a material with ultrahigh nickel content considered a strong contender for high-energy-density lithium-ion battery cathodes. We introduced nano-Al2O3 during the lithiation process to achieve dual modified material through bulk phase element doping and in situ LiAlO2 coating. Comparison revealed notable improvements in the modified materials. In particular, LiNi0.99Al0.01O2 maintained a capacity retention rate of 73.1% after 300 cycles in a long-cycle test at 0.5C current density, outperforming the undoped material. In rate performance tests, the doped samples consistently exhibited higher discharge-specific capacities than that of the undoped counterpart. Notably, at a high current density of 5C, LiNi0.99Al0.01O2 exhibited a discharge-specific capacity of 101.75 mAh g–1. The results indicate that an appropriate amount of Al doping can effectively stabilize the layered structure of the cathode material and delay the irreversible phase transition from H2 to H3. Further, Al doping facilitates the formation of a LiAlO2 coating on the surface of the particles. This coating acts as a fast-ion conductor, enhancing the transport of lithium ions and reducing the erosion of the active material by the electrolyte.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


