镍催化的烯烃的区域和立体选择性氢烷基化反应:利用重氮化合物的独特反应活性作为烷化源
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
一直以来,烯烃的反应活性都很有限,以前从未有过在任何过渡金属催化框架内使用重氮化合物对其进行氢烷基化反应的实例。在本研究中,我们介绍了利用重氮化合物作为烷化剂,在镍催化下对烯烃进行区域和立体选择性加氢烷化反应。该方法的成功归功于使用了一种基于吡啶的 P,N 配体,它结合了膦配体和氮配体的优点。此外,重氮化合物中的羰基有利于形成稳定的中间体。我们的详细机理研究表明,反应是通过与重氮化合物的初始相互作用进行的,然后与烯烃发生反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
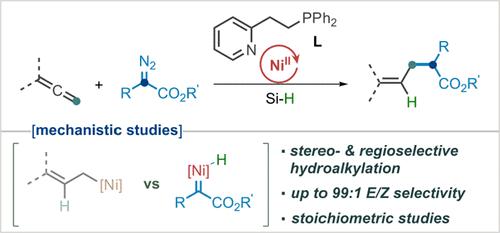
Nickel-Catalyzed Regio- and Stereoselective Hydroalkylation of Allenes: Exploiting the Unique Reactivity of Diazo Compounds as an Alkylating Source
The reactivity of allenes has historically been limited with no previous examples of their hydroalkylation using diazo compounds in any transition-metal catalytic framework. In this study, we present a Ni-catalyzed regio- and stereoselective hydroalkylation reaction of allenes utilizing diazo compounds as alkylating agents. The success of this method can be attributed to the use of a pyridine-based P,N ligand, which combines the advantages of both phosphine and nitrogen ligands. Additionally, the carbonyl group in the diazo compound facilitates the formation of stable intermediates. Our detailed mechanistic studies reveal that the reaction proceeds through an initial interaction with the diazo compound, followed by engagement with the allene.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


