塞马鲁肽治疗超重或肥胖且无糖尿病的慢性肾病患者:随机双盲安慰剂对照临床试验
IF 58.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
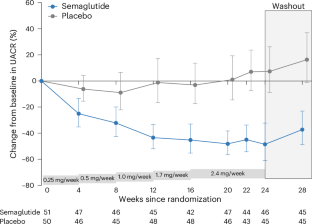
塞马鲁肽可降低2型糖尿病和慢性肾脏病(CKD)患者的白蛋白尿和肾病进展风险。我们对患有慢性肾脏病(估计肾小球滤过率(eGFR)≥25 ml min-1 1.73 m-2,尿白蛋白与肌酐比值(UACR)≥30 和 <3,500 mg g-1)且体重指数≥27 kg m-2的成人患者进行了随机安慰剂对照双盲临床试验。参与者随机接受每周2.4毫克的塞马鲁肽或安慰剂治疗。主要终点是第24周时UACR与基线相比的百分比变化。安全监测贯穿始终。总共有 125 名参与者接受了筛选,其中 101 人被随机分配到了塞马鲁肽(n = 51)或安慰剂(n = 50)。平均年龄为 55.8(s.d. 12)岁;40 名参与者(39.6%)为女性;UACR 中位数为 251 mg g-1(四分位距为 100,584);平均 eGFR 为 65.0(s.d. 25)ml min-1 1.73 m-2;平均体重指数为 36.2(s.d. 5.6)kg m-2。慢性肾小球肾炎(n = 25)和高血压性慢性肾脏病(n = 27)是最常见的慢性肾脏病病因。与安慰剂相比,使用塞马鲁肽治疗24周后,UACR降低了-52.1%(95%置信区间为-65.5, -33.4;P < 0.0001)。与安慰剂(15 例)相比,使用塞马鲁肽(30 例)更容易出现胃肠道不良事件。塞马鲁肽治疗24周后,超重/肥胖和非糖尿病性慢性肾脏病患者的白蛋白尿减少了,这在临床上很有意义。ClinicalTrials.gov 注册:NCT04889183。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Semaglutide in patients with overweight or obesity and chronic kidney disease without diabetes: a randomized double-blind placebo-controlled clinical trial
Semaglutide reduces albuminuria and the risk of kidney disease progression in patients with type 2 diabetes and chronic kidney disease (CKD). We conducted a randomized placebo-controlled double-blind clinical trial in adults with CKD (estimated glomerular filtration rate (eGFR) ≥25 ml min−1 1.73 m−2 and urine albumin-to-creatinine ratio (UACR) ≥30 and <3,500 mg g−1) and body mass index ≥27 kg m−2. Participants were randomized to semaglutide 2.4 mg per week or placebo. The primary endpoint was percentage change from baseline in UACR at week 24. Safety was monitored throughout. Overall, 125 participants were screened, of whom 101 were randomized to semaglutide (n = 51) or placebo (n = 50). Mean age was 55.8 (s.d. 12) years; 40 participants (39.6%) were female; median UACR was 251 mg g−1 (interquartile range 100, 584); mean eGFR was 65.0 (s.d. 25) ml min−1 1.73 m−2; and mean body mass index was 36.2 (s.d. 5.6) kg m−2. Chronic glomerulonephritis (n = 25) and hypertensive CKD (n = 27) were the most common CKD etiologies. Treatment for 24 weeks with semaglutide compared to placebo reduced UACR by −52.1% (95% confidence interval −65.5, −33.4; P < 0.0001). Gastrointestinal adverse events were more often reported with semaglutide (n = 30) than with placebo (n = 15). Semaglutide treatment for 24 weeks resulted in a clinically meaningful reduction in albuminuria in patients with overweight/obesity and non-diabetic CKD. ClinicalTrials.gov registration: NCT04889183 . In participants with obesity and chronic kidney disease without diabetes, once-weekly administration of semaglutide 2.4 mg led to a reduction in albuminuria, body weight and systolic blood pressure compared with placebo, with no changes to creatinine or cystatin-C estimated glomerular filtration rate or measured glomerular filtration rate during the 24-week follow-up period.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


