纯肠内营养启动个体保护性微生物组变化,诱导小儿克罗恩病病情缓解
IF 20.6
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
摘要
纯肠内营养(EEN)是治疗小儿克罗恩病(CD)的一线疗法,但其保护机制尚不清楚。我们建立了一个前瞻性儿科队列,以描述对 EEN(德国临床试验 DRKS00013306)无效的 CD 患者粪便微生物群的功能和代谢物变化。综合多组学分析从个体可变的微生物组图谱中发现了网络集群,Lachnospiraceae 和中链脂肪酸是保护性特征。生物正交非规范氨基酸标记可选择性地识别对中链脂肪酸有反应的细菌物种。元基因组分析确定了对 EEN 反应的高菌株动态。通过肠道恒温培养和将微生物群转移到无菌的Il10缺陷小鼠体内,进一步验证了饮食暴露粪便微生物群的功能变化。饮食模型条件诱导了患者特异性菌株特征,以预防或导致非生物小鼠发生类似炎症性肠病(IBD)的炎症。因此,我们提供的证据表明,EEN疗法是通过明确改变时间和个体可变的微生物组特征来发挥作用的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
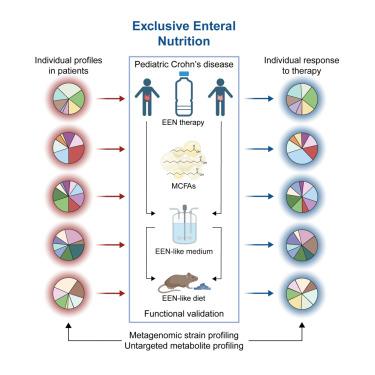
Exclusive enteral nutrition initiates individual protective microbiome changes to induce remission in pediatric Crohn’s disease
Exclusive enteral nutrition (EEN) is a first-line therapy for pediatric Crohn’s disease (CD), but protective mechanisms remain unknown. We established a prospective pediatric cohort to characterize the function of fecal microbiota and metabolite changes of treatment-naive CD patients in response to EEN (German Clinical Trials DRKS00013306). Integrated multi-omics analysis identified network clusters from individually variable microbiome profiles, with Lachnospiraceae and medium-chain fatty acids as protective features. Bioorthogonal non-canonical amino acid tagging selectively identified bacterial species in response to medium-chain fatty acids. Metagenomic analysis identified high strain-level dynamics in response to EEN. Functional changes in diet-exposed fecal microbiota were further validated using gut chemostat cultures and microbiota transfer into germ-free Il10-deficient mice. Dietary model conditions induced individual patient-specific strain signatures to prevent or cause inflammatory bowel disease (IBD)-like inflammation in gnotobiotic mice. Hence, we provide evidence that EEN therapy operates through explicit functional changes of temporally and individually variable microbiome profiles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


