使用 PEDOT:PSS 电极对原代细胞进行高效电穿孔
IF 11.7
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
将大分子精确、高效地输送到细胞中,可促进基础生物学研究以及细胞疗法、药物输送和个性化医疗方面的治疗应用。脉冲电场电穿孔能有效渗透细胞膜,无需使用有毒的化学或病毒转导剂就能递送有效载荷,但传统的散装电穿孔设备在细胞存活率和异质性方面面临着重大挑战,这是因为跨细胞产生的电场和电极-电解质界面的电化学存在差异。在这里,我们介绍了基于掺杂聚苯乙烯磺酸盐的导电聚合物聚(3,4-亚乙二氧基噻吩)(PEDOT:PSS)的微细加工电极的使用,这种电极可大幅提高细胞存活率和转染效率。作为概念验证,我们展示了向细胞系和原代细胞输送 Cas9 蛋白、引导 RNA 和质粒 DNA 的增强效果。使用 PEDOT:PSS 可以快速改造难以转染的细胞类型,从而加快研究并将其用作治疗平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
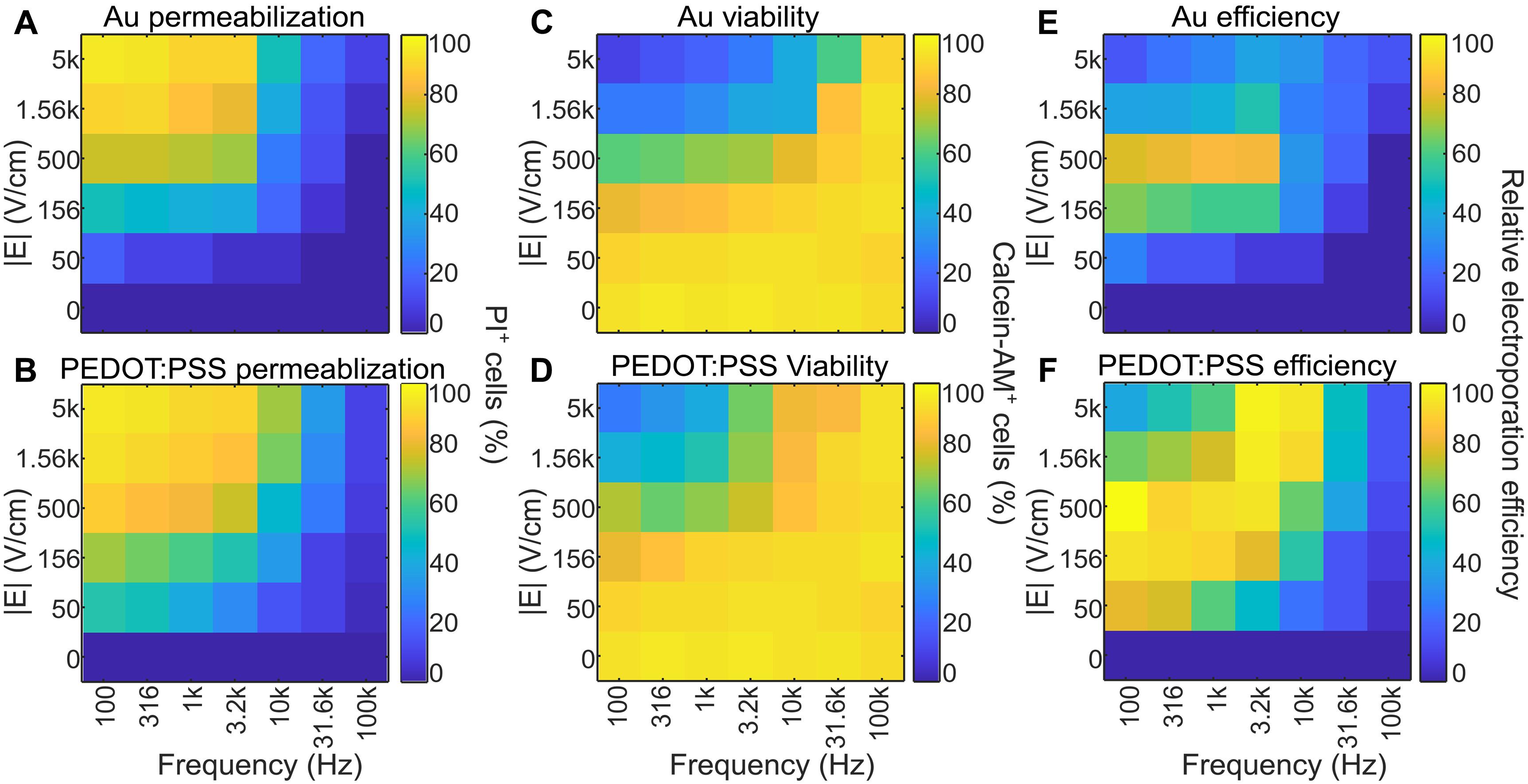
Efficient electroporation in primary cells with PEDOT:PSS electrodes
Precise and efficient delivery of macromolecules into cells enhances basic biology research and therapeutic applications in cell therapies, drug delivery, and personalized medicine. While pulsed electric field electroporation effectively permeabilizes cell membranes to deliver payloads without the need for toxic chemical or viral transduction agents, conventional bulk electroporation devices face major challenges with cell viability and heterogeneity due to variations in fields generated across cells and electrochemistry at the electrode-electrolyte interface. Here, we introduce the use of microfabricated electrodes based on the conducting polymer poly(3,4-ethylenedioxythiophene) doped with polystyrene sulfonate (PEDOT:PSS), which substantially increases cell viability and transfection efficiency. As a proof of concept, we demonstrate the enhanced delivery of Cas9 protein, guide RNA, and plasmid DNA into cell lines and primary cells. This use of PEDOT:PSS enables rapid modification of difficult-to-transfect cell types to accelerate their study and use as therapeutic platforms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


