高分子量全生物基半芳香族呋喃聚酰胺的合成及其聚合工艺研究
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
生物基单体呋喃-2,5-二羧酸(FDCA)可替代对苯二甲酸实现生物基半芳香族聚酰胺的合成,但FDCA存在因脱羧而难以提高聚合物分子量的问题,从而影响材料性能。因此,本研究采用了一种以 FDCA 酯化产物(DMFD)为原料合成高分子量半芳香族聚酰胺的方法。虽然这种方法降低了单体的活性,但提高了单体的稳定性,是目前常用的方法。因此,我们选择了能通过亲核机理提高反应活性的胺基催化剂。以 2-羟基吡啶为催化剂,通过熔融聚合反应合成了聚五亚甲基呋喃酰胺(PA5F)系列。研究了不同聚合阶段的产物结构,并对反应进行了推断。结果表明,具有亲核能力的吡啶催化剂在聚合早期提高了 DMFD 的聚合活性,同时避免了低聚物末端氨基的 N 甲基化。同时,研究还发现,在吡啶催化剂作用下进行酯胺聚合的过程中,聚合物的末端酯基会发生反应并转化为羧基。这将在高温聚合后期引起脱羧反应,造成端基比例失调,导致端基封端,限制分子量的增加。最后,通过优化反应工艺配方,在进料中加入过量(15 摩尔 %)的五亚甲基二胺(PMD),可以聚合出 Mw 为 150,000 克/摩尔的高分子量聚酰胺材料。研究结果表明,以 2-羟基吡啶为代表的吡啶催化剂在制备高分子量半芳香族聚酰胺方面具有巨大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
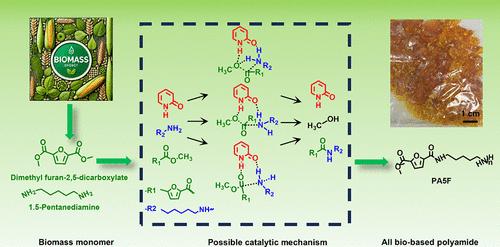
Synthesis of High-Molecular-Weight All-Biobased Semiaromatic Furan Polyamide and Study on Its Polymerization Process
Biobased monomer furan-2,5-dicarboxylic acid (FDCA) can replace terephthalic acid to achieve the synthesis of biobased semiaromatic polyamides, but FDCA has the problem of difficulty in increasing polymer molecular weight due to decarboxylation, which affects material properties. Hence, this study employed a method of synthesizing high-molecular-weight semiaromatic polyamides using FDCA esterification products (DMFD) as raw materials. Although this method reduces the activity of monomers, it improves the stability of monomers and is currently a commonly used method. Therefore, amine-based catalysts that enhance the reaction activity through nucleophilic mechanisms are chosen. A poly(pentamethylene furan amide) (PA5F) series was synthesized by melt polymerization using 2-hydroxypyridine as a catalyst. The product structures at different polymerization stages were studied, and the reaction was inferred. The results showed that pyridine catalysts with a nucleophilic ability improved the polymerization activity of DMFD in the early polymerization stage while avoiding N-methylation of the terminal amino group of the oligomers. At the same time, it was revealed that in the process of ester amine polymerization under pyridine catalysts, the end ester groups of the polymer will react and transform into carboxyl groups. This causes a decarboxylation reaction in the later stage of high-temperature polymerization, resulting in an imbalanced end-group ratio, leading to end-group sealing and limiting the molecular weight increase. Finally, by optimizing the reaction process formula and adding an excess (15 mol %) of pentamethylene diamine (PMD) to the feed, a high-molecular-weight polyamide material with Mw of 150,000 g/mol can be polymerized. The results of this study indicate that pyridine catalysts represented by 2-hydroxypyridine have great potential in the preparation of high-molecular-weight semiaromatic polyamides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


