低维混合金属卤化物半导体中的远程手性转移
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在混合金属卤化物包光体中,手性特性通常来自于结构对称性的破坏,即在结构上加入手性 A 位有机阳离子,这可能会限制组成空间。在这里,我们展示了高效的远程手性转移,即通过近端手性分子将手性强加给原本非手性的混合卤化金属半导体,该分子并不作为结构的一部分穿插其中,但却能产生高达 10-2 的大圆二色性不对称因子 (gCD)。密度泛函理论计算显示,立体化学信息从手性近端分子到无机框架的传递是通过与二价金属阳离子的选择性相互作用进行的。手性分子的锚定会引起中心不对称变形,这种变形在金属卤化物晶格的四个无机层中都可以看到。这一概念广泛适用于具有不同维度(一维和二维)的低维混合金属卤化物,可独立控制手性的组成和程度。本文章由计算机程序翻译,如有差异,请以英文原文为准。
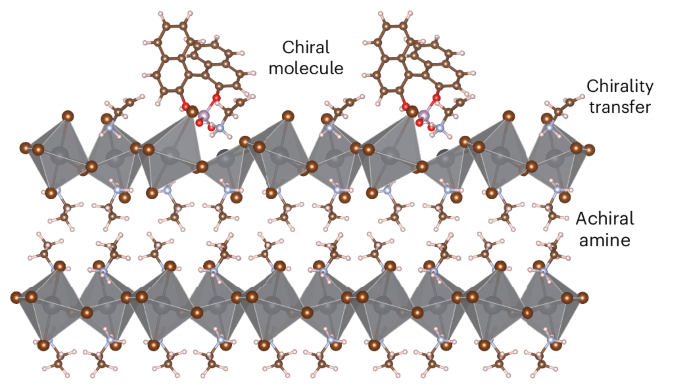

Remote chirality transfer in low-dimensional hybrid metal halide semiconductors
In hybrid metal halide perovskites, chiroptical properties typically arise from structural symmetry breaking by incorporating a chiral A-site organic cation within the structure, which may limit the compositional space. Here we demonstrate highly efficient remote chirality transfer where chirality is imposed on an otherwise achiral hybrid metal halide semiconductor by a proximal chiral molecule that is not interspersed as part of the structure yet leads to large circular dichroism dissymmetry factors (gCD) of up to 10−2. Density functional theory calculations reveal that the transfer of stereochemical information from the chiral proximal molecule to the inorganic framework is mediated by selective interaction with divalent metal cations. Anchoring of the chiral molecule induces a centro-asymmetric distortion, which is discernible up to four inorganic layers into the metal halide lattice. This concept is broadly applicable to low-dimensional hybrid metal halides with various dimensionalities (1D and 2D) allowing independent control of the composition and degree of chirality. Hybrid metal halide semiconductors typically rely on chiral A-site ammonium cations for chiral induction in the lattice. Now it has been shown that chirality in low-dimensional achiral metal halide semiconductors can be induced by non-ammonium, non-A-site chiral molecules through remote stereocontrol of the inorganic framework.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


