具有保留核糖体结合和抗菌活性的立体随机化肿瘤球蛋白
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
我们最近的研究表明,使用外消旋氨基酸进行固相肽合成可以得到立体随机化的肽,这些肽包含所有可能的非对映异构体,是可通过高效液相色谱法纯化的同质单质产物,而且立体随机化可调节膜破坏性环状和线性抗菌肽(AMPs)及树枝状聚合物的活性、毒性和稳定性。在这里,我们以抑制细菌核糖体的富脯氨酸 AMP oncocin 为例,测试了立体随机化是否可能与目标结合肽兼容。多达九个 C 端残基的立体随机化保留了核糖体结合和抗菌效果,包括对耐药细菌的活性,并防止血清降解。令人惊讶的是,完全立体随机化的oncocin与L-oncocin一样,在稀释生长介质中具有刺激肽吸收的活性,尽管它不与核糖体结合,这表明它具有另一种作用机制。这些实验表明,立体随机化可与目标结合肽兼容,并有助于了解它们的作用机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
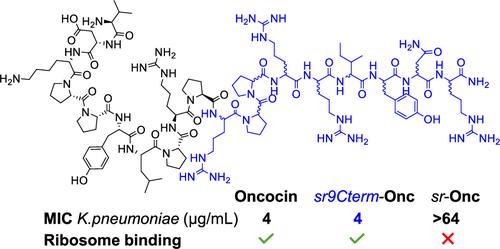
Stereorandomized Oncocins with Preserved Ribosome Binding and Antibacterial Activity
We recently showed that solid-phase peptide synthesis using racemic amino acids yields stereorandomized peptides comprising all possible diastereomers as homogeneous, single-mass products that can be purified by HPLC and that stereorandomization modulates activity, toxicity, and stability of membrane-disruptive cyclic and linear antimicrobial peptides (AMPs) and dendrimers. Here, we tested if stereorandomization might be compatible with target binding peptides with the example of the proline-rich AMP oncocin, which inhibits the bacterial ribosome. Stereorandomization of up to nine C-terminal residues preserved ribosome binding and antibacterial effects including activities against drug-resistant bacteria and protected against serum degradation. Surprisingly, fully stereorandomized oncocin was as active as L-oncocin in dilute growth media stimulating peptide uptake, although it did not bind the ribosome, indicative of an alternative mechanism of action. These experiments show that stereorandomization can be compatible with target binding peptides and can help understand their mechanism of action.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


