发现治疗纤维化的高选择性构象型齐聚物整合素 αvβ6 抑制剂 MORF-627
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
抑制整合素αvβ6是治疗特发性肺纤维化等纤维化疾病的一种很有前景的方法。通过筛选一个小型化合物库,将能稳定整合素 αIIbβ3 弯曲封闭构象的头部基团与 αv 整合素结合基团相结合,发现了能结合 αvβ6 弯曲封闭构象的热门化合物。这些化合物与 αvβ6 及相关整合素结合的晶体结构揭示了提高药效和选择性的机会,利用精确的自由能扰动 (FEP+) 计算加速了这些工作。通过优化 PK 参数(包括渗透性、生物利用度、清除率和半衰期),发现了候选药物 MORF-627,这是一种高选择性的 αvβ6 抑制剂,能稳定弯曲封闭构象,具有良好的口服 PK。遗憾的是,该化合物在一项为期 28 天的 NHP 安全性研究中显示出毒性,因此无法进一步开发。不过,MORF-627 是研究整合素 αvβ6 生物学的有用工具化合物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
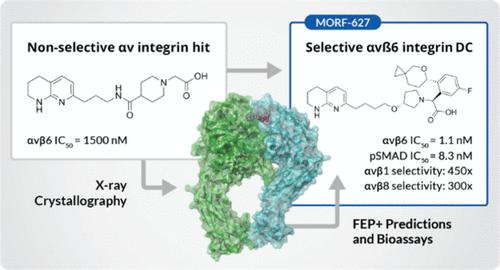
The Discovery of MORF-627, a Highly Selective Conformationally-Biased Zwitterionic Integrin αvβ6 Inhibitor for Fibrosis
Inhibition of integrin αvβ6 is a promising approach to the treatment of fibrotic disease such as idiopathic pulmonary fibrosis. Screening a small library combining head groups that stabilize the bent-closed conformation of integrin αIIbβ3 with αv integrin binding motifs resulted in the identification of hit compounds that bind the bent-closed conformation of αvβ6. Crystal structures of these compounds bound to αvβ6 and related integrins revealed opportunities to increase potency and selectivity, and these efforts were accelerated using accurate free energy perturbation (FEP+) calculations. Optimization of PK parameters including permeability, bioavailability, clearance, and half-life resulted in the discovery of development candidate MORF-627, a highly selective inhibitor of αvβ6 that stabilizes the bent-closed conformation and has good oral PK. Unfortunately, the compound showed toxicity in a 28-day NHP safety study, precluding further development. Nevertheless, MORF-627 is a useful tool compound for studying the biology of integrin αvβ6.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


