通过挖掘不同生物合成途径的杂合反应性,扩大亚胺还原酶的范围
IF 11.5
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
亚胺还原酶(IRED)是对映选择性亚胺还原和羰基化合物还原胺化的重要催化剂。然而,它们的合成多功能性受到底物范围的限制,因此需要新的 IRED。目前的 IRED 与最初表征的酶密切相关,因为它们的发现是由序列同源性搜索驱动的。在这里,我们展示了一种基于生物合成杂合性的功能基因组学方法,该方法以鉴定生物合成途径中作用于大型复杂底物的 C=N 还原酶为指导。这些底物杂化生物催化剂与现有 IRED 的同源性较低,属于不同的功能酶家族,但它们能催化非原生亚胺的氢化以及简单酮的还原胺化。因此,在没有近似同源性限制的情况下,而是在功能混杂性的指导下进一步探索序列空间,使我们发现了以前未曾认识和探索过的独特序列空间领域,以挖掘用于合成的 IRED。本文章由计算机程序翻译,如有差异,请以英文原文为准。
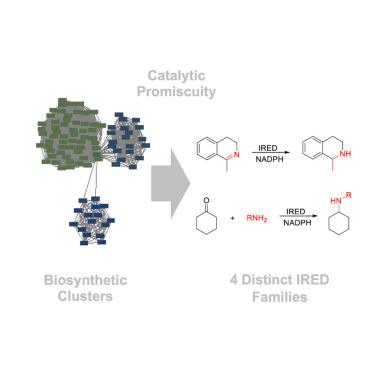
Expanding the repertoire of imine reductases by mining divergent biosynthetic pathways for promiscuous reactivity
Imine reductases (IREDs) are invaluable catalysts for enantioselective imine reduction and reductive amination of carbonyl compounds. Their synthetic versatility is, however, limited by their substrate scope, and new IREDs are needed. Current IREDs are closely related to the initially characterized enzymes, as their discovery has been driven by sequence homology searches. Here, we demonstrate a functional genomics approach based on biosynthetic promiscuity, guided by the identification of C=N reducing enzymes acting on large, complex substrates in biosynthetic pathways. These substrate-promiscuous biocatalysts share low homology to existing IREDs and fall into distinct functional enzyme families, yet they catalyze the hydrogenation of non-native imines as well as the reductive amination of simple ketones. Venturing further into sequence space without the constraints of close homology, but instead guided by functional promiscuity, has thus led us to distinct, previously unrecognized and unexplored areas of sequence space for mining IREDs for synthesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


