海马 CA3 区记忆支持神经元动力学机制
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
海马 CA3 是记忆形成和检索的核心。尽管人们提出了各种网络机制,但缺乏直接证据。通过在行为小鼠体内进行细胞内Vm记录和光遗传学操作,我们发现CA3位置场活动是由CA3锥体神经元之间的递归突触上的对称形式的行为时标突触可塑性(BTSP)产生的,而不是由来自齿状回(DG)的突触产生的。其他操作显示,根据动物的运动更新位置细胞的活动需要来自内侧皮层(EC)而非 DG 的兴奋性输入。这些数据被一个计算模型所捕获,该模型使用 BTSP 和外部更新输入来产生在线学习条件下的吸引子动力学。理论分析进一步凸显了这种网络的超强记忆存储能力,尤其是在处理相关输入模式时。这些证据阐明了海马学习和记忆形成的细胞和电路机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
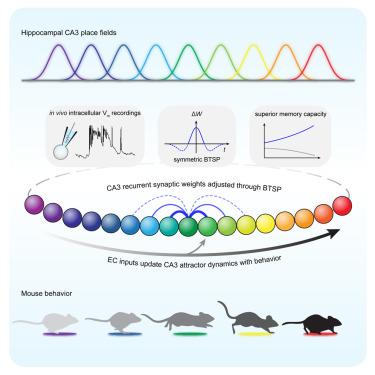
Mechanisms of memory-supporting neuronal dynamics in hippocampal area CA3
Hippocampal CA3 is central to memory formation and retrieval. Although various network mechanisms have been proposed, direct evidence is lacking. Using intracellular Vm recordings and optogenetic manipulations in behaving mice, we found that CA3 place-field activity is produced by a symmetric form of behavioral timescale synaptic plasticity (BTSP) at recurrent synapses among CA3 pyramidal neurons but not at synapses from the dentate gyrus (DG). Additional manipulations revealed that excitatory input from the entorhinal cortex (EC) but not the DG was required to update place cell activity based on the animal’s movement. These data were captured by a computational model that used BTSP and an external updating input to produce attractor dynamics under online learning conditions. Theoretical analyses further highlight the superior memory storage capacity of such networks, especially when dealing with correlated input patterns. This evidence elucidates the cellular and circuit mechanisms of learning and memory formation in the hippocampus.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


