凝胶球蛋白切断和覆盖肌动蛋白丝的机制
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
Gelsolin 是 Ca2+ 激活的 F-肌动蛋白切断和封顶蛋白家族的典型成员。我们在此报告了F-肌动蛋白倒钩末端与Ca2+结合的人类凝胶溶蛋白的结构。其中一个结构显示凝胶溶蛋白的六个结构域(G1G6)和结构域间连接器缠绕在 F-肌动蛋白上,而另一个结构显示了结构域 G1G3--一个在细胞凋亡过程中观察到的片段--结合在 F-肌动蛋白的两侧。G1G6 在 F-肌动蛋白的一侧,而 G1G3 则在两侧,它们的构象发生了变化,从而引发了断裂。Gelsolin 在断裂后保持结合,阻止亚基交换。本文章由计算机程序翻译,如有差异,请以英文原文为准。

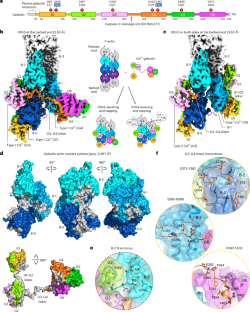
Mechanism of actin filament severing and capping by gelsolin
Gelsolin is the prototypical member of a family of Ca2+-activated F-actin severing and capping proteins. Here we report structures of Ca2+-bound human gelsolin at the barbed end of F-actin. One structure reveals gelsolin’s six domains (G1G6) and interdomain linkers wrapping around F-actin, while another shows domains G1G3—a fragment observed during apoptosis—binding on both sides of F-actin. Conformational changes that trigger severing occur on one side of F-actin with G1G6 and on both sides with G1G3. Gelsolin remains bound after severing, blocking subunit exchange. The authors use cryo-electron microscopy to reveal two structural states of Ca2+-activated gelsolin bound to the actin filament, illuminating the mechanisms of filament severing and barbed end capping.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


