苹果酸脱氢酶 2 对胶质母细胞瘤干细胞表转录组的代谢调控
IF 27.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
肿瘤会对其新陈代谢进行重编程,从而产生复杂的肿瘤生态系统。在这里,我们证明胶质母细胞瘤(GBM)干细胞(GSCs)显示出苹果酸-天门冬氨酸穿梭酶(MAS)活性升高和苹果酸脱氢酶 2(MDH2)的表达。基因和药物靶向MDH2可减轻GSC的增殖、自我更新和体内肿瘤生长,部分可被天门冬氨酸所挽救。靶向 MDH2 会诱导α-酮戊二酸(αKG)的积累,而α-酮戊二酸是二氧化酶(包括 N6-甲基腺苷(m6A)RNA 去甲基化酶 AlkB 同源物 5、RNA 去甲基化酶 ALKBH5)的重要辅助因子。强制表达 MDH2 会增加 m6A 的水平并抑制 ALKBH5 的活性,而补充 αKG 后两者均可恢复。反过来,以 MDH2 为靶标可降低全球 m6A 水平,血小板衍生生长因子受体-β(PDGFRβ)是受调控的转录本。药理抑制GSCs中的MDH2可增强口服多激酶抑制剂达沙替尼的疗效,包括PDGFRβ。总之,干样肿瘤细胞对其新陈代谢进行重编程,诱导其表转录组发生变化,并揭示了可能的治疗范例。本文章由计算机程序翻译,如有差异,请以英文原文为准。
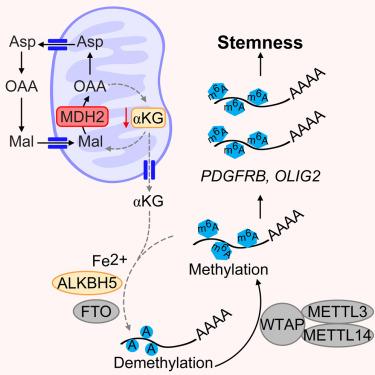
Metabolic regulation of the glioblastoma stem cell epitranscriptome by malate dehydrogenase 2
Tumors reprogram their metabolism to generate complex neoplastic ecosystems. Here, we demonstrate that glioblastoma (GBM) stem cells (GSCs) display elevated activity of the malate-aspartate shuttle (MAS) and expression of malate dehydrogenase 2 (MDH2). Genetic and pharmacologic targeting of MDH2 attenuated GSC proliferation, self-renewal, and in vivo tumor growth, partially rescued by aspartate. Targeting MDH2 induced accumulation of alpha-ketoglutarate (αKG), a critical co-factor for dioxygenases, including the N6-methyladenosine (m6A) RNA demethylase AlkB homolog 5, RNA demethylase (ALKBH5). Forced expression of MDH2 increased m6A levels and inhibited ALKBH5 activity, both rescued by αKG supplementation. Reciprocally, targeting MDH2 reduced global m6A levels with platelet-derived growth factor receptor-β (PDGFRβ) as a regulated transcript. Pharmacological inhibition of MDH2 in GSCs augmented efficacy of dasatinib, an orally bioavailable multi-kinase inhibitor, including PDGFRβ. Collectively, stem-like tumor cells reprogram their metabolism to induce changes in their epitranscriptomes and reveal possible therapeutic paradigms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


