生物碳酸钙致密液相的形成、化学演变和凝固
IF 38.5
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
金属碳酸盐在近地表矿物记录中无处不在,是生物矿化的主要产物,也是捕捉人为二氧化碳排放的重要目标。然而,由于无序前体(如致密液相(DLP))的参与,碳酸盐矿化的路径通常与经典预测不同,但人们对 DLP 的形成或凝固过程知之甚少。利用原位方法,我们报告了一种高度水合的重碳酸盐 DLP 通过液-液相分离形成,并转化为中空的水合无定形 CaCO3 颗粒。酸性蛋白质和聚合物可延长 DLP 的寿命,同时保持路径和化学性质不变。分子模拟表明,DLP 是通过溶解的 Ca²+⋅(HCO3-)2 复合物直接凝结形成的,这些复合物在封闭的 DLP 液滴中因邻近效应而发生反应。我们的研究结果深入揭示了通过液-液相分离介导的 CaCO3 成核过程,提高了直接碳酸盐矿化的能力,并阐明了一种经常被提出的复杂的生物矿化途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
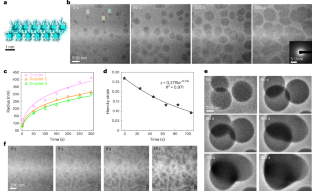

Formation, chemical evolution and solidification of the dense liquid phase of calcium (bi)carbonate
Metal carbonates, which are ubiquitous in the near-surface mineral record, are a major product of biomineralizing organisms and serve as important targets for capturing anthropogenic CO2 emissions. However, pathways of carbonate mineralization typically diverge from classical predictions due to the involvement of disordered precursors, such as the dense liquid phase (DLP), yet little is known about DLP formation or solidification processes. Using in situ methods we report that a highly hydrated bicarbonate DLP forms via liquid–liquid phase separation and transforms into hollow hydrated amorphous CaCO3 particles. Acidic proteins and polymers extend DLP lifetimes while leaving the pathway and chemistry unchanged. Molecular simulations suggest that the DLP forms via direct condensation of solvated Ca²+⋅(HCO3−)2 complexes that react due to proximity effects in the confined DLP droplets. Our findings provide insight into CaCO3 nucleation that is mediated by liquid–liquid phase separation, advancing the ability to direct carbonate mineralization and elucidating an often-proposed complex pathway of biomineralization. In situ and computational methods are used to investigate the nature of the dense liquid phase of calcium carbonate and its transformation to the amorphous phase that is common in biomineralization processes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Materials
工程技术-材料科学:综合
CiteScore
62.20
自引率
0.70%
发文量
221
审稿时长
3.2 months
期刊介绍:
Nature Materials is a monthly multi-disciplinary journal aimed at bringing together cutting-edge research across the entire spectrum of materials science and engineering. It covers all applied and fundamental aspects of the synthesis/processing, structure/composition, properties, and performance of materials. The journal recognizes that materials research has an increasing impact on classical disciplines such as physics, chemistry, and biology.
Additionally, Nature Materials provides a forum for the development of a common identity among materials scientists and encourages interdisciplinary collaboration. It takes an integrated and balanced approach to all areas of materials research, fostering the exchange of ideas between scientists involved in different disciplines.
Nature Materials is an invaluable resource for scientists in academia and industry who are active in discovering and developing materials and materials-related concepts. It offers engaging and informative papers of exceptional significance and quality, with the aim of influencing the development of society in the future.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


