通过动态动力学结晶选择性合成 N6,3′,5′-三特戊酰腺苷和区域选择性制备新戊酰化 2′-脱氧-2′-氟阿拉伯腺苷
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
在我们的 STING(干扰素基因刺激器)计划中,为制备乌洛托品,开发了从腺苷制备 2′-脱氧-2′-氟-N6,3′-二特戊酰阿拉比腺苷和 2′-脱氧-2′-氟-N6-特戊酰阿拉比腺苷的四步化学方法。这一四步路线基于腺苷的选择性保护和所需 N6,3′,5′-三特戊酰腺苷的动态结晶。随后,2′-醇被活化为其三酸盐,而不会发生特戊酸盐迁移。随后,在温和的条件下用氟化物置换三酯。对酯进行选择性脱保护可得到各种单乙酰化和二乙酰化产物,包括在 N6-特戊酰胺存在下的 3′-或 5′-保护的 2′-氟阿拉伯核苷。本文章由计算机程序翻译,如有差异,请以英文原文为准。
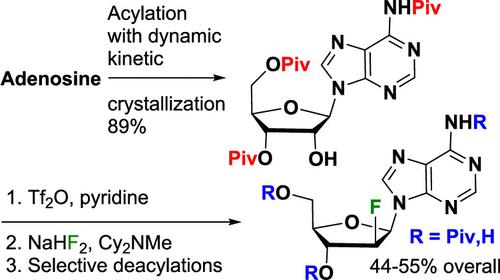
Selective Synthesis of N6,3′,5′-Tripivaloyladenosine via Dynamic Kinetic Crystallization and Regioselective Preparation of Pivalated 2′-Deoxy-2′-fluoroarabinoadenosines
A four chemical step route to 2′-deoxy-2′-fluoro-N6,3′-dipivaloylarabinoadenosine and 2′-deoxy-2′-fluoro-N6-pivaloylarabinoadenosine from adenosine was developed for the preparation of ulevostinag in our STING (Stimulator of Interferon Genes) program. This 4-step route is based on the selective protection of adenosine with a dynamic kinetic crystallization of the desired N6,3′,5′-tripivaloyladenosine. This is followed by activation of the 2′-alcohol as its triflate without pivalate migration. Subsequently, the triflate is displaced with fluoride under mild conditions. Selective deprotection of the esters can give a variety of mono- and diacylated products including the 3′- or 5′-protected 2′-fluoroarabinonucleoside in the presence of the N6-pivalamide.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


