一种时空受控、线粒体靶向的硫化氢原药可实现轻度线粒体解偶联以防止脂质沉积
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
轻度线粒体解偶联可通过调节细胞能量代谢,为肥胖症等多种疾病提供治疗益处。然而,诱导线粒体靶向轻度解偶联的有效化学干预工具非常有限。在此,我们开发了一种线粒体靶向 H2S 原药 M1,它具有按需光活化生成 H2S 的独特特性,并伴有用于实时跟踪的自报告荧光。经光照射后,M1 在线粒体中分解,生成 H2S 和一种开启荧光的香豆素衍生物,用于可视化和量化 H2S。经证实,M1 能诱导依赖活性氧(ROS)的轻度线粒体解偶联,激活线粒体相关的单磷酸腺苷激活蛋白激酶(AMPK),从而抑制棕榈酸(PA)诱导的肝细胞脂质沉积。M1诱导的解偶联功能在线粒体中受到严格控制,是通过增加细胞能量消耗来防止脂质沉积和改善代谢综合征的一种新策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
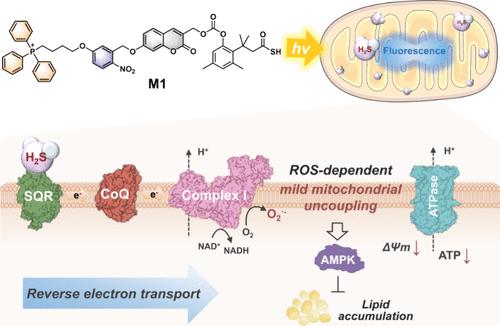
A Spatiotemporally Controlled and Mitochondria-Targeted Prodrug of Hydrogen Sulfide Enables Mild Mitochondrial Uncoupling for the Prevention of Lipid Deposition
Mild mitochondrial uncoupling offers therapeutic benefits for various diseases like obesity by regulating cellular energy metabolism. However, effective chemical intervention tools for inducing mild mitochondria-targeted uncoupling are limited. Herein, we have developed a mitochondria-targeted H2S prodrug M1 with a unique property of on-demand photoactivated generation of H2S accompanied by self-reporting fluorescence for real-time tracking. Upon photoirradiation, M1 decomposes in mitochondria to generate H2S and a turn-on fluorescent coumarin derivative for the visualization and quantification of H2S. M1 is confirmed to induce reactive oxygen species (ROS)-dependent mild mitochondrial uncoupling, activating mitochondria-associated adenosine monophosphate-activated protein kinase (AMPK) to suppress palmitic acid (PA)-induced lipid deposition in hepatocytes. The uncoupling functions induced by M1 are strictly controlled in mitochondria, representing a fresh strategy to prevent lipid deposition and improve metabolic syndrome by increasing cellular energy expenditure.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


