解除阳离子对一氧化碳电还原的影响
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
电化学二氧化碳和一氧化碳还原反应的性能受到电解液中碱金属阳离子存在的影响,但其机理仍存在争议。现在,通过实验确定了铜上电化学 CO 还原反应中关键基本步骤的能量和动力学障碍,从而得以解构阳离子效应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
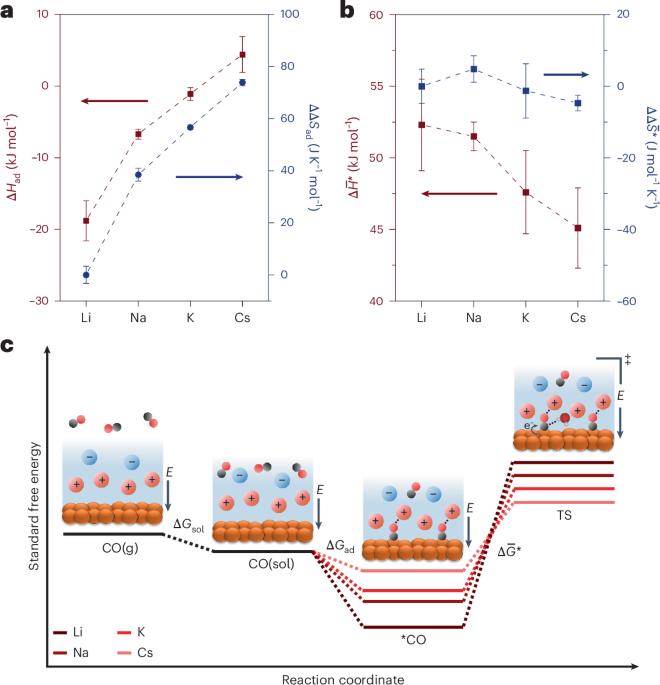
Deconvoluting the cation effect on carbon monoxide electroreduction
The performance of the electrochemical CO2 and CO reduction reactions is affected by the presence of alkali metal cations in the electrolyte, but the mechanism remains under debate. Now, experimental determination of the energetics and kinetic barriers of key elementary steps in the electrochemical CO reduction reaction on Cu enables deconvolution of the cation effect.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


