处理复杂问题
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
现在,Bortz、Bennett 和 Fasan 在《化学》(Chem)杂志上报告说,他们利用细胞色素 P450 位点选择性 C-H 氧功能化作为关键步骤,通过化学酶 DOS(ceDOS)策略,安装功能基团作为引导支架化学转化的把手,生成复杂的小分子化合物库。研究小组使用了脂肪酸羟化酶 P450BM3 的变体,这些变体先前被设计用于将倍半萜内酯 parthenolide (PTL) 转化为 9(S)-hydroxy-PTL、14-hydroxy-PTL 和 1,10-epoxy-PTL。通过酸催化重排和(大)环化、开环或闭环偏合成反应、氧化和酯化、1,4-加成以及对已安装的二烯烃进行 Diels-Alder 环加成反应等方法,将立体选择性安装的官能团作为化学处理剂,使 PTL 发生多样性转化。总体而言,这种结构多样化的努力产生了一个由 51 种化合物组成的化合物库。化学信息学工具证实,该化合物库的多样性和复杂性都有所提高,其所包含的化学空间大于更常见的后期 C-H 功能化策略所能达到的空间。此外,原理验证试验显示了针对癌细胞系的一系列生物活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
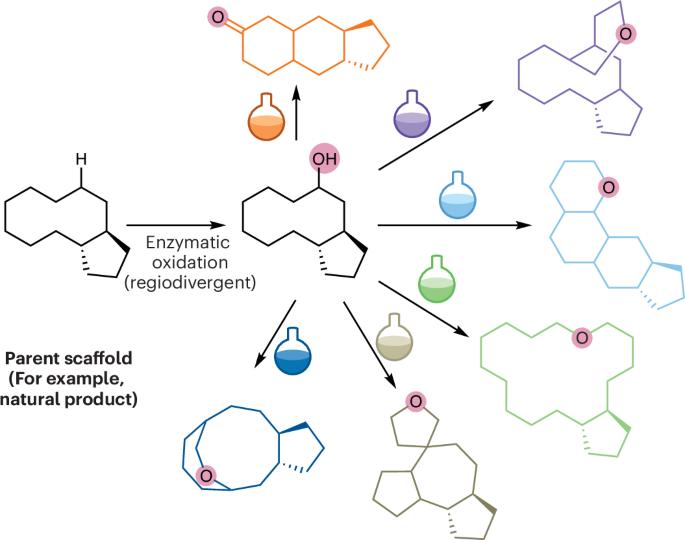
Handles for complexity building
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


