管内吹气
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
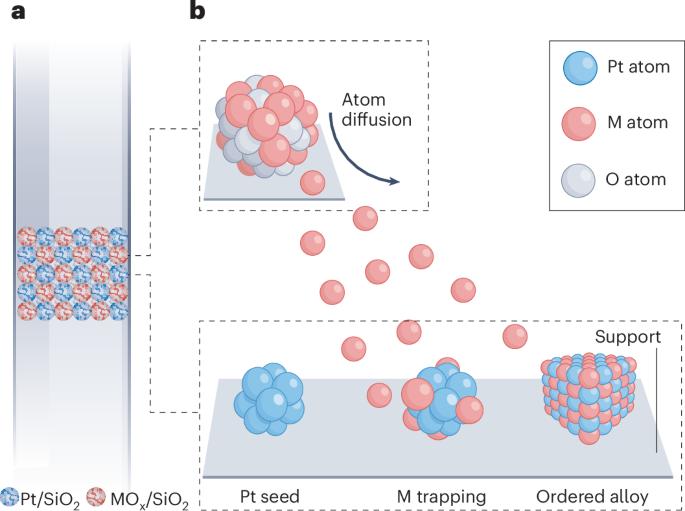
该方法的基础是为目标金属间化合物的每个元素使用二氧化硅支撑的金属前驱体。研究小组首先选择了铂锌合金,并相应地通过初湿浸渍法制备了 Pt/SiO2 和 ZnO/SiO2 前驱体。这些前驱体在氢气环境下进行物理混合并在流动反应器中加热(如图,a 部分),热曲线呈阶梯状。温度在 170-350 °C 之间时,铂在反应环境下首先被还原,并形成成核点(如图,面板 b)。因此,温度升高到 350 ℃ 以上时,氧化锌被还原,同时移动的锌原子迁移到铂种子上,形成有序的金属间铂锌合金。在光谱特性分析的指导下,对合成参数进行了优化,最终形成了具有特定成分的合金--PtZn1.4/SiO2。事实证明,这种材料是丙烷脱氢制丙烯的一种非常活跃的催化剂,在工业相关条件下具有很高的选择性。由于采用了直接的再生方法,这种催化剂还能在超过 1300 小时的使用过程中保持良好的耐久性。研究小组还将这种原子气体迁移方法扩展到制备其他可用于丙烷脱氢的金属间催化剂,包括铂-镓和铂-铟。这种方法的通用性以及制备有序结构的可能性,使原子气体迁移方法成为催化界的一个有趣工具。成功制备出能用于其他气固反应的金属间合金以及放大合成所需材料的能力仍是未来研究的课题,以证明这种合成策略的最终潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Blowing in the tube
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


