封装在氧化茚酸盐通道中的离散 Ru(Sn)6 八面体:具有高度有序的 In/Sn 位点的 RuSn6In6O16
IF 7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
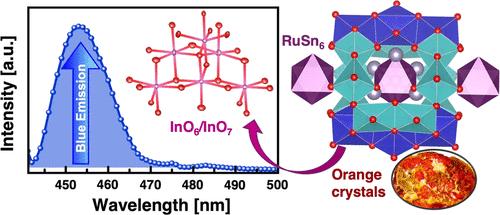
通过锡通量法制备了一种新的过渡金属混合主族元素团簇化合物 RuSn6In6O16。单晶结构细化结果表明,RuSn6In6O16 在单斜晶系中结晶,具有中心对称的 C2/m 空间群。它显示出一种独特的结构类型,让人联想到金属有机框架(MOF)结构。该结构展示了一个高正向的[Ru(Sn)6]14+金属团簇(客体),它被包裹在一个由InO6/InO7多面体的边角共享组合形成的氧化铟酸盐框架(宿主)中。经实验和理论测定,金属团簇中的氧化态为 Ru2+ 和 Sn2+,氧化层中的氧化态为 In3+。该金属簇显示出与半导体材料类似的直接带隙,带状结构计算也证实了这一点。光致发光光谱在 455 纳米处显示出一个峰值(蓝色发射),这可能源于微晶粉末中的氧空位。这表明 RuSn6In6O16 有潜力用于发光二极管(LED)和光伏应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Discrete Ru(Sn)6 Octahedra Encapsulated in Oxoindate Channels: RuSn6In6O16 with Highly Ordered In/Sn Sites
A new transition metal–mixed main group element cluster compound, RuSn6In6O16, was prepared via the tin-flux method. Single-crystal structure refinements show that RuSn6In6O16 crystallizes in a monoclinic crystal system with a centrosymmetric C2/m space group. It shows a unique structure type reminiscent of metal–organic framework (MOF) structures. The structure exhibits a highly positive [Ru(Sn)6]14+ metallic cluster (guest) encapsulated in an oxoindate framework (host) formed by a combination of corner- and edge-sharing InO6/InO7 polyhedra. The oxidation states were experimentally and theoretically determined to be Ru2+ and Sn2+ in the metallic cluster and In3+ in the oxide layers. The cluster shows a direct band gap like that of semiconducting materials, which was also confirmed by band structure calculations. The photoluminescence spectrum exhibits a peak at 455 nm (blue emission), which may originate from oxygen vacancies in the microcrystalline powder. This indicates that RuSn6In6O16 has the potential to be used in light-emitting diodes (LEDs) and photovoltaic applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


