中空 Ni@S-1 的表面重组促进了化学循环中 CH4 和 CO2 的活化
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
化学循环重整 CH4 和还原 CO2 是生产合成气和利用 CO2 的有效技术。本研究的重点是中空 Ni@S-1 的表面重组,它与 LaFe0.8Co0.15Cu0.05O3 包晶氧化物相结合,从而获得了一种用于化学循环的高效氧载体。镍浸渍硅灰石-1 经四丙基氢氧化铵处理后,采用 "溶解-重结晶 "法形成了具有空心结构的 Ni@S-1。镍最初以植硅酸镍的形式存在于 Ni@S-1 中。经过 CH4 氧化后,植硅酸镍转化为镍颗粒,并被包裹在 S-1 中,从而有效抑制了镍颗粒的烧结。在 CH4 氧化阶段,20 个氧化还原循环期间,LFCC/15%Ni@S-1 的合成气产率从 8.25 mmol-g-1 微降至 7.84 mmol-g-1,显示出比纯 LFCC 更好的稳定性和反应活性。经过 20 次氧化还原循环后,Fe 和 Co 元素从透辉石迁移到 Ni@S-1,表明透辉石和 Ni@S-1 之间的相互作用更强,这可能有利于反应活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
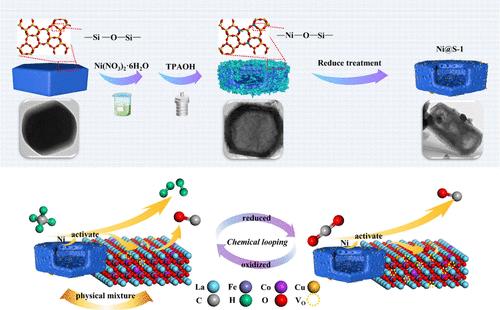
Surface Restructuring of Hollow Ni@S-1 Promoted the Activation of CH4 and CO2 in Chemical Looping
Chemical looping reforming of CH4 coupled with the reduction of CO2 is an effective technology for syngas production and CO2 utilization. This work focuses on the surface restructuring of hollow Ni@S-1, which combines with LaFe0.8Co0.15Cu0.05O3 perovskite oxides to obtain a highly efficient oxygen carrier for chemical looping. Ni-impregnated silicalite-1 was subjected to treatment with tetrapropylammonium hydroxide, resulting in the formation of Ni@S-1 with a hollow structure using the “dissolution-recrystallization” method. Nickel is initially present in the form of nickel phyllosilicate in Ni@S-1. After CH4 oxidation, nickel phyllosilicates are transformed into Ni particles that are encapsulated into S-1, effectively suppressing the sintering of Ni particles. In the CH4 oxidation stage, the syngas yield over the LFCC/15%Ni@S-1 decreases slightly from 8.25 to 7.84 mmol·g–1 during 20 redox cycles, demonstrating better stability and reactivity than that of pure LFCC. After 20 redox cycles, Fe and Co elements migrate from perovskite to Ni@S-1, indicating a stronger interaction between perovskite and Ni@S-1, which may benefit the reactivity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


