转录因子 TCF1 与 RORγt 结合,协调决定 Th17 细胞平衡状态的调控网络
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
T辅助(Th)17细胞包含一系列细胞状态,包括维持组织稳态功能的细胞和可导致自身免疫组织损伤的促炎症细胞。确定决定 Th17 细胞状态的调节因子可以找出控制组织炎症和恢复平衡的方法。在这里,我们发现白细胞介素(IL)-23(一种对诱导促炎Th17细胞至关重要的细胞因子)会降低转录因子T细胞因子1(TCF1)的表达。即使在没有IL-23受体信号传导的情况下,成熟T细胞中TCF1的条件性缺失也会增加Th17细胞的促炎潜能,并使处于平衡状态的Th17细胞具有促炎潜能。相反,TCF1的持续表达会降低Th17细胞的促炎潜能。从机理上讲,TCF1与RORγt结合,从而干扰了其促炎功能,并协调了一个决定Th17细胞状态的调控网络。我们的研究结果确定了TCF1是Th17细胞状态的主要决定因素,并为开发治疗Th17驱动的炎症性疾病的疗法提供了重要启示。本文章由计算机程序翻译,如有差异,请以英文原文为准。
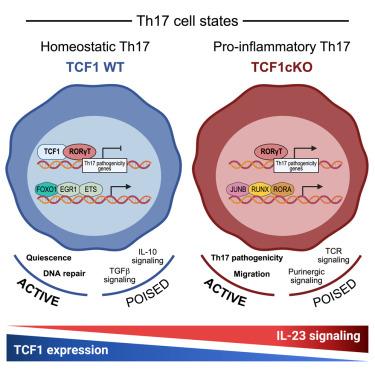
Transcription factor TCF1 binds to RORγt and orchestrates a regulatory network that determines homeostatic Th17 cell state
T helper (Th) 17 cells encompass a spectrum of cell states, including cells that maintain homeostatic tissue functions and pro-inflammatory cells that can drive autoimmune tissue damage. Identifying regulators that determine Th17 cell states can identify ways to control tissue inflammation and restore homeostasis. Here, we found that interleukin (IL)-23, a cytokine critical for inducing pro-inflammatory Th17 cells, decreased transcription factor T cell factor 1 (TCF1) expression. Conditional deletion of TCF1 in mature T cells increased the pro-inflammatory potential of Th17 cells, even in the absence of IL-23 receptor signaling, and conferred pro-inflammatory potential to homeostatic Th17 cells. Conversely, sustained TCF1 expression decreased pro-inflammatory Th17 potential. Mechanistically, TCF1 bound to RORγt, thereby interfering with its pro-inflammatory functions, and orchestrated a regulatory network that determined Th17 cell state. Our findings identify TCF1 as a major determinant of Th17 cell state and provide important insight for the development of therapies for Th17-driven inflammatory diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


