利用高通量电化学对低功率细菌维持状态进行机理研究
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
由于缺乏可操作的实验系统,对生命代谢低限的机理研究一直很有限。在这里,我们发现铜绿假单胞菌(Pseudomonas aeruginosa)对酚嗪-1-甲酰胺(PCN)的氧化还原循环支持细胞在不生长的情况下进行维持,在 25°C 温度条件下的低质量代谢率为 8.7 × 10-4 W (g C)-1。利用高通量电化学培养装置,我们发现循环 PCN 的非生长细胞能耐受常规抗生素,但对针对膜成分的抗生素敏感。在这些条件下,细胞通过依赖醋酸激酶和 NADH 脱氢酶的非典型、促进性发酵来保存能量。在限制细胞存活的 PCN 浓度范围内,细胞特异性代谢率保持恒定,这表明细胞在接近其生物能极限的条件下运行。这种定量平台为进一步研究维持生理状态的机理打开了大门,这种生理状态是微生物在自然界和疾病中生存的基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
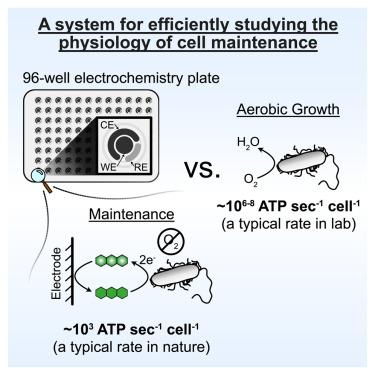
Mechanistic study of a low-power bacterial maintenance state using high-throughput electrochemistry
Mechanistic studies of life’s lower metabolic limits have been limited due to a paucity of tractable experimental systems. Here, we show that redox-cycling of phenazine-1-carboxamide (PCN) by Pseudomonas aeruginosa supports cellular maintenance in the absence of growth with a low mass-specific metabolic rate of 8.7 × 10−4 W (g C)−1 at 25°C. Leveraging a high-throughput electrochemical culturing device, we find that non-growing cells cycling PCN tolerate conventional antibiotics but are susceptible to those that target membrane components. Under these conditions, cells conserve energy via a noncanonical, facilitated fermentation that is dependent on acetate kinase and NADH dehydrogenases. Across PCN concentrations that limit cell survival, the cell-specific metabolic rate is constant, indicating the cells are operating near their bioenergetic limit. This quantitative platform opens the door to further mechanistic investigations of maintenance, a physiological state that underpins microbial survival in nature and disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


