利用无膜电化学系统从富含硝酸盐的废水中回收氨氮
IF 25.7
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
电催化硝酸盐还原法在同时实现氨回收和富含硝酸盐的废水处理方面具有巨大潜力。然而,长期以来,废水基质的复杂性阻碍了其在废水处理行业的实施和商业化。在此,我们开发了一种无膜电化学系统,称为与 NH3 回收同步的电化学 NO3- 转化系统(ECSN),它能使硝酸盐还原与氨回收同步进行,以处理实际的富硝酸盐废水。该系统的关键部件包括具有良好耐腐蚀性能的 3D 打印金属玻璃装饰铜镍(MPCN)工作电极和紫外线辅助剥离装置。在处理实际电镀废水时,ECSN 系统可将 70% 以上的硝酸盐转化为高纯度氯化氨。长期稳定性测试证明了 ECSN 系统在处理实际废水时的稳定性。此外,技术经济分析和生命周期分析也证明了该系统的经济可行性和环境效益。总之,这项工作使电催化硝酸盐还原工艺离实际应用更近了一步,为环境保护和人为氮流的循环做出了贡献。通过电化学硝酸盐还原法回收废水中的氨将支持循环经济。在此,作者开发了一种无膜电化学系统,可从富含硝酸盐的实际废水中高效、稳健地回收氨。本文章由计算机程序翻译,如有差异,请以英文原文为准。
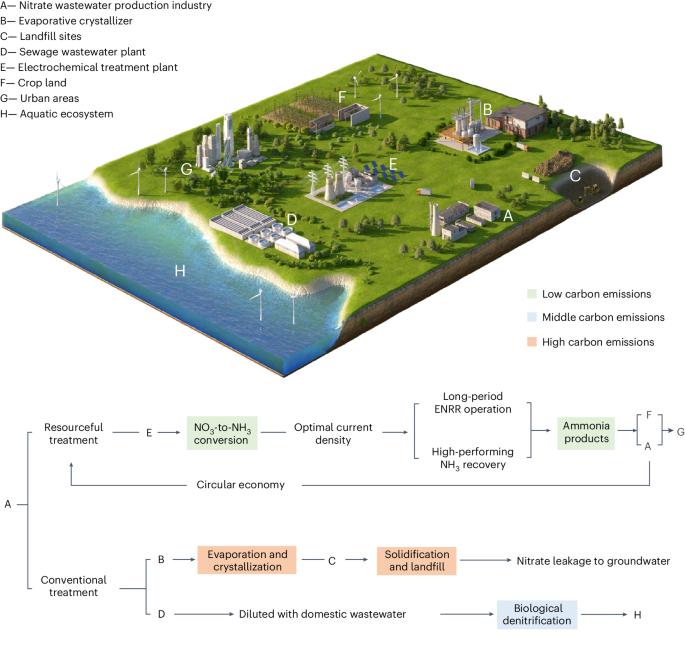
Ammonia recovery from nitrate-rich wastewater using a membrane-free electrochemical system
Electrocatalytic nitrate reduction has great potential for simultaneously achieving ammonia recovery and nitrate-rich wastewater treatment. However, the complexity of wastewater matrices has long hampered its implementation and commercialization in the wastewater treatment industry. Here we develop a membrane-free electrochemical system, called electrochemical NO3− conversion synchronized with NH3 recovery (ECSN), which synchronizes nitrate reduction with ammonia recovery for treating real nitrate-rich wastewater. Key components of this system include a 3D-printed metallic glass decorated Cu–Ni (MPCN) working electrode bearing good corrosion resistance and a UV-assisted stripping unit. When treating real electroplating wastewater, the ECSN system converts over 70% of nitrate into high-purity ammonia chloride. Long-term stability test demonstrates the robustness of the ECSN system in treating real wastewater. Further, the economic feasibility and environmental benefits of this system are evidenced by technoeconomic analysis and life-cycle analysis. Overall, this work brings the electrocatalytic nitrate reduction process one step closer to practical application, contributing to both environmental protection and the circularity of anthropogenic nitrogen flow. Recovering ammonia from wastewater via electrochemical nitrate reduction would support a circular economy. Here the authors develop a membrane-free electrochemical system that allows efficient and robust ammonia recovery from real nitrate-rich wastewater.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Sustainability
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
41.90
自引率
1.10%
发文量
159
期刊介绍:
Nature Sustainability aims to facilitate cross-disciplinary dialogues and bring together research fields that contribute to understanding how we organize our lives in a finite world and the impacts of our actions.
Nature Sustainability will not only publish fundamental research but also significant investigations into policies and solutions for ensuring human well-being now and in the future.Its ultimate goal is to address the greatest challenges of our time.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


