纳米粒子介导的协同破坏肿瘤神经支配和氧化还原平衡的强效抗肿瘤疗法
IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
神经支配与多种促进肿瘤生长的生物过程密切相关,因此成为越来越有前景的治疗靶点。本研究开发了伪装有肿瘤细胞膜的仿生空心二氧化锰纳米载体(HMLC),以包裹神经支配抑制剂利多卡因,从而实现有效的抗肿瘤治疗。这种方法旨在抑制神经纤维的生长,诱导细胞内氧化还原失衡。得益于肿瘤 "归巢 "效应,HMLC 会在血液循环过程中积聚在肿瘤组织中,并通过同源膜融合被肿瘤细胞内吞。进入细胞后,MnO2 可被过度产生的谷胱甘肽和 H2O2 降解,从而导致 Mn2+ 和利多卡因的肿瘤特异性释放。Mn2+ 介导的芬顿样反应会促进活性氧的积累,由此产生的氧化应激加上谷胱甘肽的耗竭会加剧氧化还原失衡。同时,释放的利多卡因会下调神经生长因子和神经蛋白。神经生长因子的减少会明显抑制肿瘤组织中神经纤维的形成和浸润,而神经素的减少则会降低细胞内 Ca2+,从而有助于防止转移。总之,这一策略凸显了基于纳米粒子的肿瘤神经支配干扰物在抗肿瘤治疗中的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
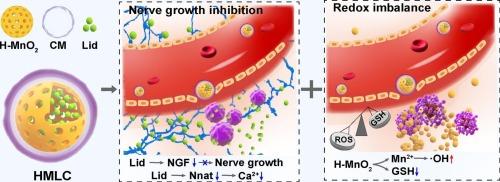
Nanoparticle-mediated synergistic disruption of tumor innervation and redox homeostasis for potent antineoplastic therapy
Innervation is closely linked to several biological processes that promote tumor growth, making it an increasingly promising therapeutic target. In this study, biomimetic hollow MnO2 nanocarriers camouflaged with tumor cell membranes (HMLC) are developed to encapsulate lidocaine, an innervation inhibitor, for effective antineoplastic therapy. This approach aims to suppress nerve fiber growth and induce intracellular redox imbalance. Benefiting from the tumor-homing effect, HMLC accumulates in cancerous tissue during circulation and is endocytosed by tumor cells through homologous membrane fusion. Once inside the cells, MnO2 can be degraded by the overproduced glutathione and H2O2, leading to the tumor-specific release of Mn2+ and lidocaine. The Mn2+-mediated Fenton-like reaction promotes the accumulation of reactive oxygen species, and the resulting oxidative stress, combined with glutathione depletion, exacerbates redox imbalance. Simultaneously, the released lidocaine downregulates nerve growth factor and neuronatin. The reduction in nerve growth factor significantly inhibits nerve fiber formation and infiltration in tumor tissue, while the decrease in neuronatin reduces intracellular Ca2+, which helps prevent metastasis. Overall, this strategy highlights the potential of nanoparticle-based tumor innervation disruptors in antineoplastic therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


